Points to Remember: Coordination Chemistry | Inorganic Chemistry PDF Download
Points to be Remembered
1. Double Salt vs. Complex Salt:
2. Werner's Theory: The basic postulates of Werner's coordination theory are given below:
- Each metal has two valencies - Primary valency and Secondary valency,
- Primary valency is called ionizable valency and it corresponds to the oxidation no. of metal. It is satisfied by the oppositely charged ions.
- Secondary valency is called non-ionizable valency and it corresponds to coordination no. of a metal, It is satisfied by same, opposite and neutral species.
- The species which satisfy secondary valency of metal are called ligands.
- Secondary valency is directional while primary valency is non-directional.
- The ligands has lone pairs. These lone pairs are donated to the metal by ligands in a compound. These are called coordination compounds.
3. Classification of Ligands:
I. Classification Based on the no. of Donor Centres:
- Mono-dentate Ligand: The ligands having only one donor centre are called mono-dentate ligands. Example - NH3, H2O etc.
- Bi-dentate Ligand: The ligands having two donor centre are called bi-dentate ligands. Example - Ethylene glycol etc.
- Hexa-dentate Ligand: The ligands having six donor centres are called hexa-dentate ligands. Example - Ethylene diamine tetra acetate (EDTA).
II. Classification Based on the Mode of Electron Donation:
- Only σ-donor Ligands: The ligands which donates electrons to the metal through only σ- donation pathway are called σ-donor ligands. Example - CH3- etc.
- Both σ-donor and π-donor Ligands: The ligands which donates electrons to the metal through both a as well as σ-donation pathway are called a as well as n-donor ligands. Example - HO- etc.
- σ-Donor but π-acceptor Ligands: The ligands which donates electrons to the metal by σ-donation pathway but accept electron from metal through π- back acceptance pathway. Example - CO, PF3 etc.
- Ligands Having π-electrons but Donated as σ-electrons: The ligands which contains rr-electrons but during donation to the metal it seems to be o- electrons. Example - Ethylene.
III. Ambident Ligand: The ligand which contains two different donor centres but donates through only one centre at a time is called ambident ligand. Example - CN- has two donor centres - C(soft centre) and N(hard centre).
IV. Flexidentate Ligand: The ligand which has many donor centres but it can use sometime only one donor atom for coordination, sometimes two donor atoms for coordination or, sometimes all of its donor atoms for coordination is called flexidentate ligand. Example - EDTA.
V. Chelated Ligand: The ligand which undergo small and stable ring formation (5/6/7 membered) is called Chelated ligand. The chelation is due to the entropy effect. Example - Ethylene glycol can form five membered Chelated ring with metal. Hence, it is a Chelated ligand.
4. Stepwise Formation Constant vs. Overall Formation Constant: Let us consider the following reactions:
M + L = ML ; K1 = [ML]/{[M][L]}
ML + L = ML2 ; K2 = [M L2]/{[M L][L]}
ML2 + L = ML3 ; K3 = [ML3]/[[ML2][L]}
where, K1; K2 and K3 are stepwise formation constants respectively.
Also, M + 3L= ML3 ; β3 = [ML3]/{ [M][L ]3}
Now, β3 = [ML3]/{[M][L]3}
= {[ML3]/[ML2].[L]}. {[ML2]/[ML] [L]}. {[ML]/[M] [L]}
= K3.K2.K1.
Hence, βn = K1,K2,K3.....Kn
5. Successive Formation Constants Gradually Decreases: It is observed that the successive formation constants values decrease. This is due to the following reasons:
- Columbic Factor: At first metal stays positively charged. Hence, it will have high appetite for negatively charged ligand. After the incorporation of the first ligand, the negative charge density on the metal is decreased. Hence, it will have lower appetite for the incorporation of another new ligand. Similarly, after the attachment of the second ligand, the electron deficiency on the metal is decreased much more. Hence, the appetite for the incorporation o f the third ligand by the metal will be much lesser. In the same way the successive formation constants will be decreased.
- Steric Factor: As the small water molecules are replaced by bulkier ligands, the steric crowding in the molecule after each ligand incorporation will be increased. Hence, the rate of successive ligands incorporation will be decreased. Hence, the successive formation constants will be decreased.
- Orientation Factor: In case of a octahedral complex, the first ligand incorporation can occur from any direction of the molecule. But, the last ligand incorporation will only occur from one direction. Hence, the chance of replacement of water molecule by fresh ligand will be much less resulting in the very low rate constant.
6. Inner-orbital Complex (LS) vs. Outer-orbital Complex (HS): Formation of LS or, HS complex depends on the following factor
- Charge on Metal: Greater is charge on metal smaller will be the size of the metal. Hence, more closer a ligand can approach to the metal. Hence, higher will be chance of formation of LS complex.
- Effect of Orbital: On moving from 3d to 4d orbital containing metal, due to increase of effective nuclear charge the ligand-metal interaction will be increased. Hence, higher will be the chance of LS complex formation.
- Nature of Ligands: Grater is the strength of a ligand, higher will be the ligand-metal interaction and hence, higher will be the chance of formation of LS complex. CO, CN- etc. are called strong field ligands. F-, HO- etc.are called weak field ligand. NH3 etc. are called moderate field ligand.
7. Jahn-Teller Distortion: It states that any non-linear molecule having degenerate electronic state will undergo distortion to remove degeneracy and to reduce energy. Strong J-T distortion occurs due to unsymmetrical distributions of electrons in eg orbital while weak J-T distortion will occur due to the unsymmetrical distribution of electrons in t2g orbital.In case, of K4[Cr(CN)6](A) : It is a LS com plex as the ligand CN- is a strong field ligand. Cr is present as Cr2+. Hence, its electronic configuration will be: t2g4eg°. As the t2g orbital is unsymmetrically occupied, it will have weak Jahn-Teller distortion.
In case of K4[Fe(CN)6](B): It is also a LS complex as the ligand CN- is a strong field ligand. Fe is present as Fe2+. Hence, its electronic configuration will be: t2g6eg°. As the t2g orbital is symmetrically occupied, it will have no Jahn-Teller distortion.
8. Trans Effect: The formation of cis-platin (anticancer agent) and trans-platin can be explained on the basis of trans effect. When [Pt(NH3)4]2+ is reacted with Cl-,[PtCl(NH3)3]+ will form. Now the order of trans effect of chloride and ammonia ligands are: Cl- > NH3. Hence, the incorporation of second chloride ligand will occur at the position ortho to amine and para to chloride by following the principle of greater trans influence of chloro ligand. The reaction scheme is shown below:
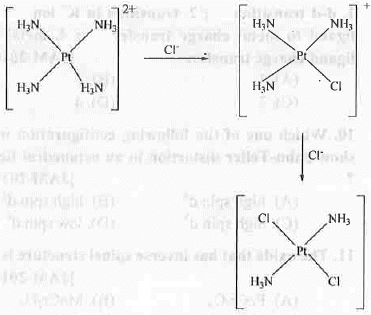 It is prepared from tetrachloroplatinate(II). Chloride ligand has greater trans effect than Ammonia ligand. That's why PtCl42- is used as the precursor for the preparation of cis-platin.
It is prepared from tetrachloroplatinate(II). Chloride ligand has greater trans effect than Ammonia ligand. That's why PtCl42- is used as the precursor for the preparation of cis-platin. 9. Colour of Coordination Compounds: Colour of a coordination compound may arise due to the two reasons - d-d transition and charge transfer transition.
9. Colour of Coordination Compounds: Colour of a coordination compound may arise due to the two reasons - d-d transition and charge transfer transition.
I. d-d Transition: Presence of d-electron(s) is the primary condition for d-d transition. But, colour intensity depends on whether the d-d transition is allowed or, it is forbidden. This is determined by two factors : 1. Symmetry allowed (Laporte allowed) and 2. Spin allowed.
II. Charge Transfer Transition: This can be of three types - A. Ligand to metal charge transfer (LMCT), B. Metal to ligand charge transfer (MLCT) and C. Intra- valent metal to metal charge transfer (IMMCT). These are shortly discussed:
- Ligand to Metal Charge Transfer (LMCT): It occurs when metal is at high oxidation state i.e. can be reducible while ligand is at high negative oxidation state i.e. can be oxidisable. In case of KMnO4, the metal is present at +7 oxidation state. Hence, it can be easily reduced while ligand is at -2 oxidation state and hence, can be easily oxidized. Hence, there will be strong ligand to metal charge transfer which falls in the visible region which will make it to appear as intense purple.
- Metal to Ligand Charge Transfer (MLCT): It occurs when metal is at low oxidation state i.e. can be oxidisable while ligand has energetically low - lying vacant orbital i.e. can be reducible. In case of [Fe(bpy)3]2+ complex, the metal is present at +2 oxidation state. Hence, it can be easily oxidized while bipyridyl system has vacant low-lying π -orbital and hence, can be easily reduced. Hence, there will beo strong metal to ligand charge transfer which falls in the visible region which will make it _ to appear as intense red.
- Intra-valent Metal to Metal Charge Transfer (IMMCT): The Prussian blue and Turn blue colour are explained by this transition. Here, Fe are present at two different oxidation states - +2 and +3.
|
50 videos|92 docs|41 tests
|
FAQs on Points to Remember: Coordination Chemistry - Inorganic Chemistry
| 1. What is coordination chemistry? |  |
| 2. What is a ligand in coordination chemistry? |  |
| 3. How are coordination compounds named? |  |
| 4. What is the coordination number in coordination chemistry? |  |
| 5. What are some applications of coordination compounds? |  |
















