NEET Exam > NEET Notes > Physics Class 11 > Mindmap: Kinetic Theory of Gases
Mindmap: Kinetic Theory of Gases | Physics Class 11 - NEET PDF Download
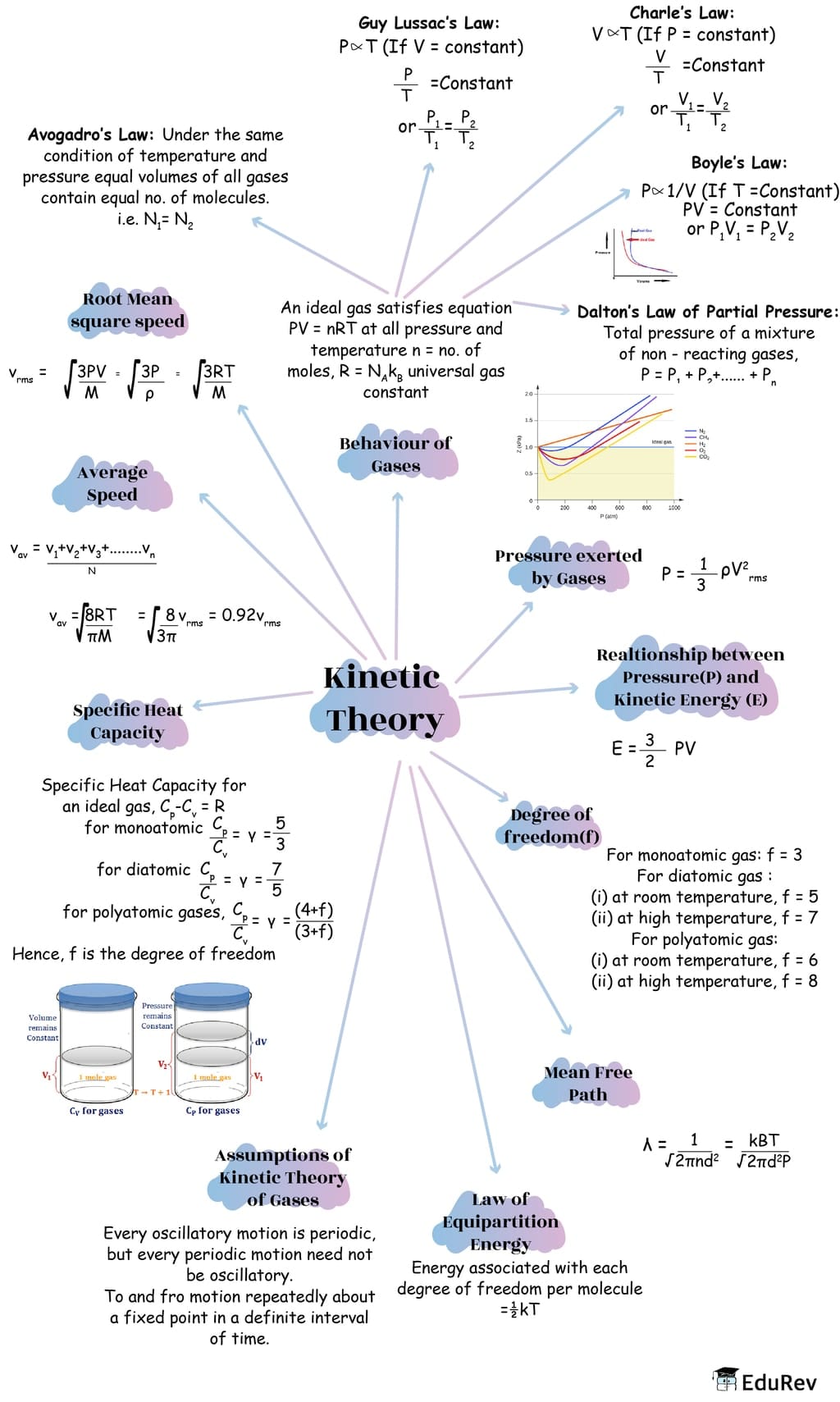
The document Mindmap: Kinetic Theory of Gases | Physics Class 11 - NEET is a part of the NEET Course Physics Class 11.
All you need of NEET at this link: NEET
|
97 videos|378 docs|103 tests
|
FAQs on Mindmap: Kinetic Theory of Gases - Physics Class 11 - NEET
| 1. What is the kinetic theory? |  |
The kinetic theory is a scientific model that explains the behavior of particles in matter. It states that all particles are in constant motion and that the temperature of a substance is proportional to the average kinetic energy of its particles.
| 2. How does the kinetic theory explain the properties of gases? |  |
The kinetic theory explains the properties of gases by stating that gas particles are in constant random motion and collide with each other and the walls of their container. These collisions create pressure, and the temperature of the gas is related to the average kinetic energy of its particles.
| 3. What is the relationship between temperature and kinetic energy according to the kinetic theory? |  |
According to the kinetic theory, temperature is directly proportional to the average kinetic energy of the particles in a substance. As the temperature increases, the particles move faster and have higher kinetic energy.
| 4. How does the kinetic theory explain the expansion of gases when heated? |  |
The kinetic theory explains the expansion of gases when heated by stating that as the gas is heated, the average kinetic energy of its particles increases. This increase in kinetic energy causes the particles to move faster and collide more frequently with each other and the walls of the container, leading to an increase in volume or expansion.
| 5. Can the kinetic theory be applied to solids and liquids as well? |  |
Yes, the kinetic theory can be applied to solids and liquids as well. While the particles in solids and liquids have less freedom to move compared to gases, they still possess kinetic energy and are in constant motion. The kinetic theory helps explain the behavior and properties of these states of matter as well.

|
Explore Courses for NEET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches


















