NCERT Exemplar Solutions: Combustion & Flame | Science Class 8 PDF Download
| Table of contents |

|
| Multiple Choice Questions |

|
| Very Short Answer Questions |

|
| Short Answer Questions |

|
| Long Answer Questions |

|
Multiple Choice Questions
Q.1. A substance which reacts with oxygen giving heat is called a combustible substance.
Which one of the following is a combustible substance?
(a) iron nail
(b) glass
(c) stone piece
(d) wood
Ans: d
Explanation:
Glass, iron nail and stone piece will not burn like wood hence they are not combustible. Wood burns in air and it is called the combustible substance.
Q.2. Which one of the following has the highest calorific value?
(a) kerosene
(b) biogas
(c) LPG
(d) petrol
Ans: c
Explanation:
Calorific represents the amount of heat generated when fuel burns completely.
The calorific value of LPG is 55000-kilo joules/ Kg, which is high when compared to the calorific value of Kerosene, Biogas and petrol.
Q.3. Magnesium ribbon on burning in air produces:
(a) magnesium oxide, water and light.
(b) magnesium oxide and heat.
(c) magnesium oxide, heat and light.
(d) magnesium oxide, water and heat.
Ans: c
Explanation:
Magnesium reacts with atmospheric oxygen to get magnesium oxide by liberating heat and light.
The chemical reaction is – Mg+O2 → MgO2 + Heat+ Light.
Q.4. Which of the following is not a combustible substance?
(a) camphor
(b) glass
(c) straw
(d) alcohol
Ans: b
Explanation:
Camphor, alcohol, straw can burn in air and they are combustible substance. Glass will not burn in air and it is non-combustible substance.
Q.5. The substance that does not burn with flame is
(a) LPG
(b) camphor
(c) dry grass
(d) charcoal
Ans: d
Explanation:
The charcoal will not vaporize on heating. Hence charcoal will not burn by producing the flame. Charcoal glows on combustion.
Q.6. On placing an inverted tumbler over a burning candle, the flame extinguishes after some time. This is because of the non-availability of
(a) oxygen
(b) water vapours
(c) carbon dioxide
(d) wax
Ans: a
Explanation:
Oxygen is necessary for combustion. When we place inverted tumbler over a burning candle, oxygen supply will be stopped, this extinguishes the flame.
Q.7. If a person’s clothes catch fire, the best way to extinguish the fire is to:
(a) throw water on the clothes.
(b) use the fire extinguisher.
(c) cover the person with a woollen blanket.
(d) cover the person with a polythene sheet.
Ans: c
Explanation:
By covering a person’s clothes with the woollen blanket we are cutting off the oxygen required for burning of clothes. Hence fire will be distinguished.
Q.8. The substance expected to have the highest ignition temperature out of the following is
(a) kerosene
(b) petrol
(c) coal
(d) alcohol
Ans: c
Explanation:
Ignition temperature is the lowest temperature at which a substance catches fire. Among the given options coal have highest ignition temperature.
Q.9. Choose the correct statement about inflammable substances from the following. They have:
(a) low ignition temperature and cannot catch fire easily.
(b) high ignition temperature and can catch fire easily.
(c) low ignition temperature and can catch fire easily.
(d) high ignition temperature and cannot catch fire easily.
Ans: c
Explanation:
The inflammable substances are the substances that easily catch fire. They are substances, which have very low ignition temperature and when exposed to air, they catch fire easily.
Q.10. Choose the incorrect statement from the following. Forest fires are usually due to:
(a) carelessness of humans
(b) heat of sun
(c) cutting of trees
(d) lightning strike
Ans: c
Explanation:
Deforestation or Cutting of trees is not the cause for the forests fire. Forest fires are usually caused due to heat of sun, lightning strike and carelessness of humans
Q.11. The calorific value of a fuel is expressed in a unit called
(a) kilojoule per litre
(b) kilogram per millilitre
(c) kilojoule per gram
(d) kilojoule per kilogram
Ans: d
Q.12. In villages, people use wood as fuel because:
(a) it is considered to be an ideal fuel.
(b) of its easy availability and low cost.
(c) it is environment-friendly.
(d) it catches fire easily.
Ans: b
Q.13. Which among the following is considered as the cleanest fuel?
(a) cow dung cake
(b) petrol
(c) kerosene
(d) hydrogen gas
Ans: d
Explanation:
Hydrogen gas is called as clean fuel as it does not produce any dangerous gases. It can only produce heat and water as products.
Q.14. Choose the incorrect statement from the following. A good fuel is one which:
(a) is readily available.
(b) produces a large amount of heat.
(c) leaves behind many undesirable substances.
(d) burns easily in the air at a moderate rate.
Ans: c
Explanation:
Good fuel is readily available and produces a large amount of heat. A good fuel burns easily in the air at a moderate rate and will not leave behind any undesirable substances.
Q.15. Shyam was cooking potato curry on a chulha. To his surprise, he observed that the copper vessel was getting blackened from outside. It may be due to:
(a) proper combustion of fuel.
(b) improper cooking of potato curry.
(c) improper combustion of the fuel.
(d) burning of the copper vessel.
Ans: c
Explanation:
The copper vessel was blackened from outside because of the insufficiency of oxygen.
Very Short Answer Questions
Q.1. Fill in the blanks in the following sentences.
(a) A ____________ process in which a substance reacts with __________ to give off heat is called combustion.
(b) When the clothes of a person catch __________, the person is covered with a __________ to extinguish fire.
(c) The __________ temperature at which a substance catches fire is called its __________ temperature.
(d) The substances which have very ________________ ignition temperature and can easily catch fire with a flame are called __________ substances.
(e) The substances which vapourise during __________, give flame.
Ans:
(a) A Chemical process in which a substance reacts with oxygen to give off heat is called combustion.
(b) When the clothes of a person catch fire, the person is covered with a blanket to extinguish the fire.
(c) The lowest temperature at which a substance catches fire is called its ignition temperature.
(d) The substances which have very low ignition temperature and can easily catch fire with a flame are called inflammable substances.
(e) The substances which vapourise during burning give flame.
Q.2. Some words (underlined) in the following sentences are jumbled up. Write them in their correct form.
(a) Seldie is a combustible substance.
(b) Slags is a non-combustible material.
(c) Chittsmack does not burn by itself.
(d) Some substances on combustion produce thea and mafel.
(e) The amount of heat energy produced on complete combustion of 1 kg of a fuel is called its Aricolci uelav.
Ans:
(a) diesel.
(b) glass.
(c) matchstick.
(d) heat, flame.
(e) calorific value.
Short Answer Questions
Q.1. Two glass jars A and B are filled with carbon dioxide and oxygen gases, respectively. In each jar, a lighted candle is placed simultaneously. In which jar will the candle remain lighted for a longer time and why?
Ans: Candle remains in jar B because it is filled with oxygen which is necessary for the combustion and flame. In Jar A candle will not burn due to lack of oxygen.
Q.2. Anu wants to boil water quickly in a test tube. On observing the different zones of the flame, she is not able to decide which zone of the flame will be best for boiling water quickly. Help her in this activity.
Ans: Anu should use the outermost zone of the flame which is the hottest part of the flame.
Q.3. Why is the use of diesel and petrol as fuels in automobiles being replaced by Compressed Natural Gas (CNG) in big cities?
Ans: Compared to petrol and diesel CNG is a clean fuel. It produces very less amount of gases which are poisonous hence in big cities CNG is used in place of petrol and diesel.
Q.4. Boojho wants to separate the following materials as combustible and non-combustible. Can you help him?
Charcoal, chalk, stone, iron rod, copper coin, straw, cardboard, glass, paper, wood.
Ans:
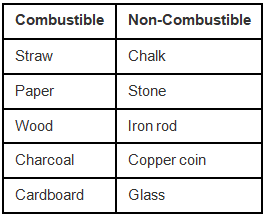
Q.5. Indicate whether the following statements are True or False. Also write the false statements in their correct form.
(a) Air is necessary for combustion.
(b) Magnesium is a non-combustible metal.
(c) Carbon dioxide is an excellent fire extinguisher.
(d) Calorific value of wood is higher than that of coal
Ans:
- True
- False. Because, Magnesium is a combustible metal.
- True
- False- Because the alorific value of coal is higher than that of wood.
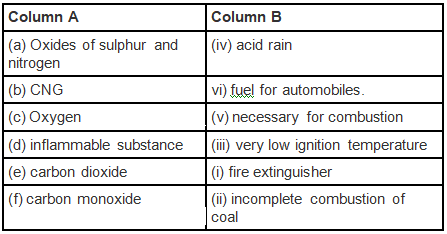
Q.6. Match the items of Column A with the items of Column B
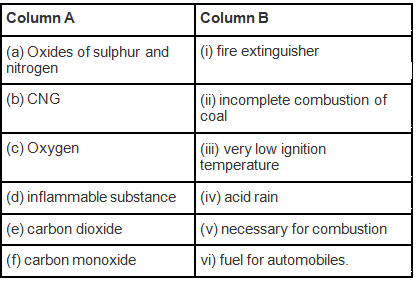
Ans:
Q.7. Match the following for the flame of a candle.
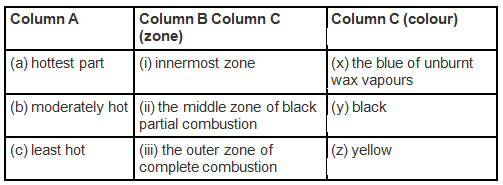
Ans:
Q.8. If you hold a piece of iron wire with a pair of tongs inside a candle flame or a Bunsen burner flame, what will you observe? Will it produce a flame?
Ans: Iron will not produce flame rather it glows red hot.
Q.9. Fill in the blanks using the words given in the box.
ignition, petrol, combustion, calorific value, combustible, inflammable
(a) A chemical process in which a substance reacts with oxygen to give off heat is called __________.
(b) Wood, paper, CNG are __________ substances.
(c) The lowest temperature at which a substance catches fire is called its __________ temperature.
(d) Ignition temperature of __________ is lower than that of wood.
(e) The substances which have very low __________ temperature and can easily catch fire with a flame are called __________ substances.
(f) The amount of heat energy produced on complete combustion of 1kg of a fuel is called its __________
Ans:
(a) A chemical process in which a substance reacts with oxygen to give off heat is called combustion.
(b) Wood, paper, CNG are combustible substances.
(c) The lowest temperature at which a substance catches fire is called its Ignition temperature.
(d) Ignition temperature of petrol is lower than that of wood.
(e) The substances which have very low Ignition temperature and can easily catch fire with a flame are called Inflammable substances.
(f) The amount of heat energy produced on complete combustion of 1kg of a fuel is called its Calorific value.
Q.10. People usually keep Angethi/burning coal in their closed rooms during winter season. Why is it advised to keep the door open?
Ans: Burning of coal produces carbon monoxide which is poisonous for the people inhaling it. If the door is closed, oxygen level will be less and carbon-monoxide level will be high. This is harmful for the person sleeping in the room. Hence the door should be closed.
Q.11. Write True/False against the following statements and also correct the false statement
- A physical process in which a substance reacts with oxygen to give off heat is called combustion.
- Water is the best extinguisher for fires involving electrical equipment.
- Alcohol, CNG and LPG are inflammable substances.
- Increased concentration of nitrogen in the air is believed to cause global warming.
- Greater the calorific value, better is the fuel.
- Middle zone is the hottest zone of a flame.
- The substances, which vapourise during burning, gives flame.
Ans:
- True.
- False- Carbon-di-oxide is the best extinguisher for fires involving electrical equipment.
- True.
- False- Increased concentration of carbon-di-oxide in the air is believed to cause global warming.
- True.
- False- Outer zone is the hottest zone of a flame.
- True.
Q.12. Cracker on ignition produces sound. Why?
Ans: Upon ignition, cracker releases gas is released out and the gas is heated by the amount of heat generated by the cracker. This lead to an explosion resulting in the production of sound.
Q.13. What do you understand by fuel efficiency?
Ans: Fuel efficiency is determined by its calorific value which is the amount of heat energy produced on complete combustion of 1 kg of fuel. The calorific value of a fuel is expressed in kJ/kg.
Long Answer Questions
Q.1. You are provided with three watch glasses containing milk, petrol and mustard oil, respectively. Suppose you bring a burning candle near these materials one by one, which material(s) will catch fire instantly and why?
Ans: Watchglass containing petrol will catch the fire first because petrol is highly combustible liquid and its calorific value is 45.8 MJ/kg. This is very less when compared to milk or mustard oil.
Q.2. Manu was heating oil to fry potato chips. The cooking oil all of a sudden caught fire; he poured water to extinguish the fire. Do you think this action was suitable? If yes, why? If not, why not? In such a condition what should Manu have done?
Ans: No, the step taken by Manu to extinguish fire from oil is not suitable. Because along with water oil also spread which will increase the flame.
Steps that should have been taken by Manu
Manu should have put the flame of the burner off and put a lid on frying pan. This cuts the contact between flame and oxygen and the flame will be gradually extinguished.
Q.3. What are the three essential requirements to produce fire? How fire extinguisher is useful for controlling the fire.
Ans: Three essential requirements to produce gas are:
- Oxygen.
- Combustible substance or fuel.
- Heta to acquire ignition temperature.
The fire extinguishers are useful for controlling the fire in the following ways:
- Fire extinguishers extinguish the fire by cutting the supply of air or by bringing down the temperature of fuel or both.
- The most common fire extinguisher is water. But waterworks only when things like wood and paper are on fire. If electrical equipment is on fire, water may conduct electricity and harm those trying to douse the fire. Water is also not suitable for fires involving oil and petrol. Do you recall that water is heavier than oil? So, it sinks below the oil, and oil keeps burning on the top.
- For fires involving electrical equipment and inflammable materials like petrol, carbon dioxide (CO2) is the best extinguisher. CO2, being heavier than oxygen, covers the fire like a blanket. Since the contact between the fuel and oxygen is cut off, the fire is controlled. The added advantage of CO2 is that in most cases it does not harm the electrical equipment.
Q.4. Give two examples each for a solid, liquid and gaseous fuel along with some important uses.
Ans:
Solid Fuels
- Coal- Is used to produce electricity through steam engines.
- Coke- Coke is used as reducing agent in the extraction of metals.
Liquid Fuels
- Petrol: Used to run small automobiles like bike and car.
- Kerosene- Used for domestic heating purpose and jet engines as fuel.
Gaseous fuel
- CNG – CNG is used to run automobiles.
- Natural Gas – It is used for industrial purpose.
Q.5. The calorific values of petrol and CNG are 45000 kJ/kg and 50,000 kJ/kg, respectively. If you have a vehicle which can run on petrol as well as CNG, which fuel will you prefer and why?
Ans: I prefer CNG over petrol because CNG is a clean fuel and it produces very less harmful gases. This will reduce the air pollution that happens due to burning of fuel. The calorific value of CNG is higher than that of petrol hence it can be economical.
Q.6. Although wood has a very high calorific value, we still discourage its use as a fuel. Explain.
Ans: This is because wood comes from the cutting of trees and forests, which affect the environment adversely. Cutting of trees results in increase of CO2 Content in the air, as a result, global warming takes place. Also burning of wood releases a lot of harmful gases including carbon-di-oxide, carbon-monoxide and sulphur-di-oxides.
Q.7. Forest fire produces a lot of air pollution. Write in brief about the reasons for forest fires.
Ans: Reasons for the forest fires are given below
- Lightning causes a forest fire.
- Campfire may also be a reason.
- Human negligence is one of the main reasons for a forest fire.
- At high temperature, sometimes dry grass catches fire which spreads throughout the forest.
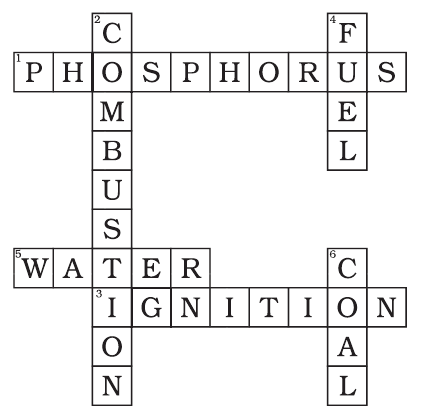
Q.8. Complete the crossword Figure with the help of the clues
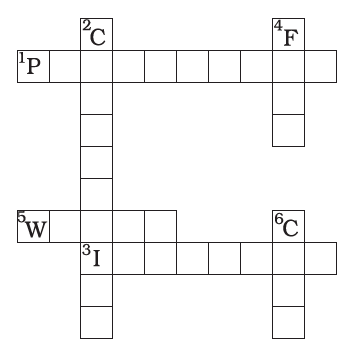
Across
1. Non-metal which catches fire if exposed to air. (10)
3. The lowest temperature at which a substance catches fire is called its …………. temperature. (8)
5. The most common fire extinguisher. (5)
Down
2. A chemical process in which a substance reacts with oxygen to give off heat. (10)
4. Petrol is used as a ………….. in automobiles. (4)
6. It is as hard as stone and black in colour. (4)
Ans:
|
92 videos|296 docs|44 tests
|
FAQs on NCERT Exemplar Solutions: Combustion & Flame - Science Class 8
| 1. What is combustion? |  |
| 2. What is the importance of combustion? |  |
| 3. What are the different types of combustion? |  |
| 4. How does combustion contribute to air pollution? |  |
| 5. How can we prevent or reduce the harmful effects of combustion? |  |




















