Chemistry Exam > Chemistry Notes > Inorganic Chemistry > General Methods of Isolation and Purification of Elements
General Methods of Isolation and Purification of Elements | Inorganic Chemistry PDF Download
General Principles and Processes of Isolation of Elements
- Minerals and Ores:
The compounds of metals found in nature along with rocky materials and other impurities are called minerals.
The minerals from which metals can be conveniently and profitably extracted are called ores. For example , minerals of aluminum are bauxite and clay. But aluminum can be extracted conveniently and economically from bauxite. Therefore , the ore of aluminum is bauxite. Thus all ores are minerals but all minerals are not ores.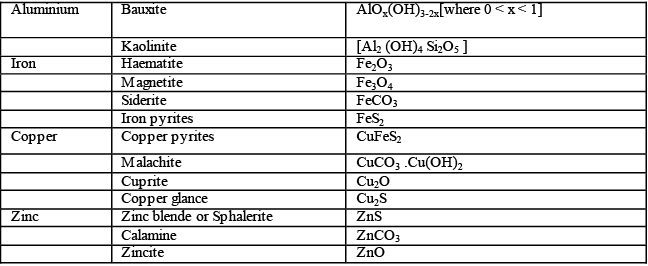
- Gangue or Matrix:
The earthy impurities associated with minerals are called gangue.
Metallurgical Processes:
The process of extraction of metals from their ores is called metallurgy. Various steps involved in the metallurgical process are;
- Concentration of ore : The process of removal of gangue from the ore is called ore dressing or ore concentration or benefication. Dep ending up on the nature of the ore and impurities one or more of methods can be used.
(i) Hydraulic Washing or Levigation: This method is used when the gangue particles are lighter than the ore particles. When the powdered ore is treated with a stream of running water, the lighter gangue particles are washed away and the heavier ore particles are left behind. E.g : cassiterite an ore of tin is purified by this method.
(ii) magnetic seperaton : When either the ore particle or the impurities are magnetic in nature, they can be separated by means of a magnetic separator. It consist s of a leather belt moving over two rollers, one of which is magnetic. The powdered ore is dropped into a hopper from where it falls on the moving belt at one end. At the other end, the magnetic portion of the ore is attracted by the magnetic roller and falls nearer to the roller while the non - magnetic impurities fall fart her off. Eg . rutile (TiO2) is concentrated by this method.
(iii) Froth Floatation Method: This method is based on the preferential wetting of the ore by oil and gangue by water. It is used for the concentration of sulphide ores which are lighter than gangue.
Here the finely powdered ore is mixed with water in a large tank. A small amount of an oil and a froth producing reagent are added to it . The whole mixture is agitated violently by passing air through it , when froth is produced. The ore particles are preferentially wetted by oil and raise to the surface along with the froth. It is skimmed off, washed and dried. The gangue particles are preferentially wetted by water and sink to the bottom of the tank. Eg : copper pyrites , zinc blende are concentrated by this method.
Sometimes, it is possible to separate two sulphide ores by using ‘depressants’. For example, in case of an ore containing ZnS and PbS, the depressant, NaCN selectively prevents ZnS from coming to the froth but allows PbS to come with the froth.
(iv) Leaching: This is a chemical method for the concentration of ores. Here, the powdered ore is treated with a reagent which can dissolve the ore but not the impurities. For example, bauxite is concentrated by this method. Bauxite, usually contains SiO2, iron oxides and titanium oxide (TiO2) as impurities. The powdered ore treated with conc. NaOH . Al2O3 is leached out as sodium aluminate
Al2O3(s) + 2NaOH(aq) + 3H2O(l) → 2Na[Al(OH)4]
The aluminate in solution is neutralised by passing CO2 gas and hydrated Al2O3 is precipitated. At this stage, the solution is seeded with freshly prepared samples of hydrated Al2O3 which induces the precipitation:
2Na[Al(OH)4 ](aq) + CO2(g) → Al2O3.xH2O(s) + 2NaHCO3 (aq)
The sodium silicate remains in the solution and hydrated alumina is filtered, dried and heated to give back pure
Al2O3 : Al2O3.xH2O(s) → Al2O3(s) + xH2O(g) - Extraction of Crude Metal from Concentrated Ore :
The concentrated ore is converted into a form which is suitable for reduction. Usually ore is converted to oxide. Thus isolation of metals from concentrated ore involves two major steps viz.,
(a) conversion to oxide : this can done either by calcination or by roasting.
i. Calcination : calcination is the process of heating the concentrated ore in a limited supply of air below its melting point. Oxygen is not used up in this process. During calcinaton the volatile matter escapes leaving behind the metal oxide: Eg:
Fe2O3 .xH2O(s) →Fe2O3(s) + xH2O(g)
ZnCO3 (s) → ZnO(s) + CO2 (g)
CaCO3 → CaO(s) + CO2 (g)
ii. Roasting: Roasting is the process of heating the concentrated ore in a good supply of air below its melting point. Oxygen is used up in this process. During roasting the volatile impurities like SO2, As2O3, P2O5 etc are expelled and the ore is converted into the oxide.
Eg : 2ZnS + 3O2 → 2ZnO + 2SO2
2PbS + 3O2 → 2PbO + 2SO2
(b) Reduction of oxide to the metal : Reduction of the metal oxide usually involves heating it with some other substance acting as a reducing agent
(C or CO or even another metal). The reducing agent (e.g., carbon) combines with the oxygen of the metal oxide.
MxOy + yC → xM + yCO
➤ Thermodynamic Principles of Metallurgy
The change in Gibbs energy, ΔG , is described by the equation: ΔG = ΔH – T ΔS
Where, ΔH is the enthalpy change and ΔS is the entropy change. Also, ΔG° = – RT ln K Where, K is the equilibrium constant of the ‘reactant – product ’ system at the temperature, T. A negative ΔG implies a +ve K in equation. And this can happen only when reaction proceeds towards products. Thus,
1. When the value of ΔG is negative, only then the reaction will proceed. If ΔS is positive, on increasing the temperature (T), the value of TΔS would increase (ΔH < TΔS) and then ΔG will become –ve.
2. If reactants and products of two reactions are put together in a system and the net ΔG of the two possible reactions is –ve, the overall reaction will occur. Such coupling is easily understood through Gibbs energy (ΔG° ) vs T plots for formation of the oxides.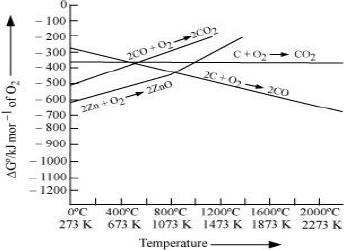
The reducing agent forms its oxide when the metal oxide is reduced. The role of reducing agent is to provide ΔG° negative and large enough to make the sum of ΔG° of the two reactions (oxidation of the reducing agent and reduction of the metal oxide) negative.
During reduction, the oxide of a metal decomposes as:
M xO(s) → xM (solid or liq) +½ O2 (g) ---------------- (1)
Reverse of the above reaction is the oxidation of the metal.
xM (s or l) +½O2 (g) → M xO(s) [ΔG° (M,MxO)] --------------- (2)
Then the oxidation of the reducing agents (e.g., C or CO) will be;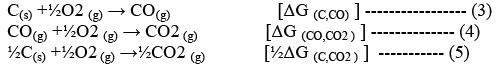
On subtracting equation (2) from one of the three equations, we get: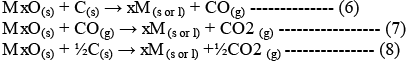
These reactions describe the actual reduction of the metal oxide, MxO.
It can be observed from the above graph that a metal can reduce the oxide of other metals, if the standard free energy of formation of the oxide of the former is more negative than the latter.
➤ Extraction of iron from its oxides:
Oxide ores of iron, after concentration through calcination /roasting are mixed with limest one and coke and fed into a Blast furnace from its top. Here, the oxide is reduced to the metal.
Reduction of oxides takes place in different zones.
(a) At 500 – 800K (lower temperature range in blast furnace)
3Fe2O3 + CO → 2Fe3O4 + CO2
Fe3O4 + 4CO → 3Fe + 4CO2
Fe2O3 + CO → 2FeO + CO2
(b) At 900 – 1500K (higher temperature range in blast furnace)
C + CO2 → 2COFeO + CO → Fe + CO2
(c) Lime stone decomposes to CaO and CO2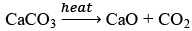
(d) Silika react with CaO to form calcium silicate which forms slag. It floats over molten iron and prevents oxidation of iron.
The iron obtained from Blast furnace contains about 4% carbon and many impurities in smaller amount. This is known as pig iron. Cast iron is different from pig iron and is made by melting pig iron with scrap iron and coke using hot air blast . It has slightly lower carbon content (about 3%) and is extremely hard and brittle.
Wrought iron is the purest form of commercial iron and is prepared from cast iron by oxidising impurities in a reverberatory furnace lined with haematite. This haematite oxidises carbon to carbon monoxide:
Fe2O3 + 3C → 2Fe + 3CO
➤ Extraction of copper from cuprous oxide:
It is easy to reduce oxide ores of copper directly to the metal by heating with coke. Cu2O + C → 2Cu + CO
However most of the ores are sulphide and some may also contain iron. The sulphide ores are roasted to give oxides:
2Cu2S + 3O2 → 2Cu2O + 2SO2
In actual process, the ore is heated in a reverberatory furnace after mixing with silica. In the furnace, iron oxide ‘slags of’ as iron silicate and copper is produced in the form of copper matte. This contains Cu2S and FeS.
FeO + SiO2 → FeSiO3 (Slag)
Copper matte is then charged into silica lined convertor. Some silica is also added and hot air blast is blown to convert the remaining FeS, FeO and Cu2S/Cu2O to the metallic copper. Following reactions take place:
2FeS + 3O2 → 2FeO + 2SO2
FeO + SiO2 → FeSiO3
2Cu2S + 3O2 → 2Cu2O + 2SO2
2Cu2O + Cu2S → 6Cu + SO2
The solidified copper obtained has blistered appearance due to the evolution of SO2 and so it is called blister copper.
➤ Extraction of zinc from zinc oxide
The reduction of zinc oxide is done using coke. The metal is distilled off and collected by rapid chilling.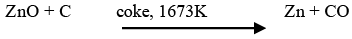
➤ Electrochemical Principles of Metallurgy
We have, ΔG0 = – nFE0
Here n is the number of electrons and E0 is the electrode potential of the redox couple formed in the system.
More reactive metals have large negative values of the electrode potential. So their reduction is difficult. If the difference of two E0 values corresponds to a positive E0 and consequently negative ΔG0, then the less reactive metal will come out of the solution and the more reactive metal will go to the solution, e.g.,
Cu2+ (aq) + Fe(s) → Cu(s) + Fe2+ (aq)
In simple electrolysis, the Mn+ ions are discharged at cathodes and deposited there.
➤ Aluminium
In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AlF6 or CaF2 and is electrolysed. Steel vessel with lining of carbon acts as cathode and graphite anode is used.
2Al2O3 + 3C → 4Al + 3CO2
This process is known as Hall-Heroult process. The oxygen liberated at anode reacts with the carbon of anode producing CO and CO2. This way for each kg of aluminium produced, about 0.5 kg of carbon anode is burnt away .
Cathode: Al3+ (melt ) + 3e– → Al(l)
Anode: C(s) + O2– (melt ) → CO(g) + 2e–
C(s) + 2O2– (melt ) → CO2 (g) + 4e–
➤ Copper from Low Grade Ores and Scraps
Copper is extracted by hydrometallurgy from low grade ores. It is leached out using acid or bacteria. The solution containing Cu2+ is treated wit h scrap iron or H2
Cu2+ (aq) + H2 (g) → Cu(s) + 2H+ (aq)
➤ Oxidation Reduction
Some extractions are based on oxidation. Example: Extraction of chlorine from brine.
2Cl– (aq) + 2H2O(l) → 2OH– (aq) + H2 (g) + Cl2 (g)
The ΔG0 for this reaction is + 422 kJ and E0 are – 2.2 V. Naturally, it will require an external e.m.f. that is greater than 2.2 V. Thus, Cl2 is obtained by electrolysis giving out H2 and aqueous NaOH as by -products. While Electrolysis of molten NaCl gives Na metal. - Refining
The process of removal of impurities from the crude metal is called refining. Several techniques are used.
(a) Distillation: This is very useful for low boiling metals like zinc and mercury. The impure metal is evaporated to obtain the pure metal as distillate.
(b) Liquation: In this method a low melting metal like tin can be made to flow on a sloping surface. In this way it is separated from higher melting impurities.
(c) Electrolytic refining: In this method, the impure metal is made to act as anode. A strip of the same metal in pure form is used as cathode. A solution of a soluble salt of the metal is used as the electrolyte. On passing electric current, the pure metal dissolves from the anode and is deposited at the cathode. The soluble impurities go in to solution. The insoluble matter settles down at the bottom and it is called anode mud or anode sludge.
Anode: M → Mn+ + ne–
Cathode: Mn+ + ne– → M
Copper is refined using this method. Anodes are of impure copper and pure copper strips are taken as cathode. CuSO4 is used as electrolyte.
Anode: Cu → Cu2+ + 2e–
Cathode: Cu2+ + 2e– → Cu
(d) Zone refining: This method is based on the principle that an impure metal on solidification will deposit crystals of pure metal and the impurities will remain behind in the molten part of the metal. In this process, heat is applied at a small region of an impure metal rod. At the heated zone the rod melts. When the heat source is slowly moved along the rod, the impurities are carried away in the molten zone to one end of the rod. The process is repeated till the desired level of purity is attained. The impurities are finally discarded. Si, Ge, B etc are purified by this method.
(e) Vapour phase refining: In this method, the metal is converted into its volatile compound. It is then decomposed to give pure metal.
Example:
Mond Process for Refining Nickel:
In this process, nickel is heated in a stream of carbon monoxide forming nickel tetracarbonyl: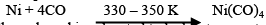
The carbonyl is subjected to higher temperature so that it is decomposed giving the pure metal: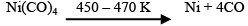
van Arkel Method for Refining Zirconium or Titanium:
In this method the metal is converted into its volatile stable compound in such a way that the impurities are not affected during compound formation. The volatile compound in them decomposed to get the pure metal. The crude metal is heated in an evacuated vessel with iodine.
Zr + 2I2 → ZrI4
The metal iodide decomposed to get the pure metal.
ZrI4 → Z r + 2I2
The document General Methods of Isolation and Purification of Elements | Inorganic Chemistry is a part of the Chemistry Course Inorganic Chemistry.
All you need of Chemistry at this link: Chemistry
|
50 videos|92 docs|41 tests
|
FAQs on General Methods of Isolation and Purification of Elements - Inorganic Chemistry
| 1. What are the general principles of isolation of elements? |  |
Ans. The general principles of isolation of elements include various methods such as reduction, electrolysis, and fractional crystallization. These methods are based on the differences in chemical and physical properties of elements and their compounds.
| 2. How is the process of isolation of elements carried out? |  |
Ans. The process of isolation of elements involves several steps. It typically begins with the extraction of the ore, followed by its concentration and purification. The purification may be done through physical or chemical methods, including filtration, distillation, and precipitation. Finally, the isolated element is obtained in its pure form.
| 3. What are the commonly used methods for the purification of elements? |  |
Ans. The commonly used methods for the purification of elements include distillation, crystallization, and electrolysis. Distillation involves the separation of a volatile component from a mixture by vaporization and subsequent condensation. Crystallization is a process in which a pure solid compound is obtained from its solution. Electrolysis is a technique that uses an electric current to drive a non-spontaneous chemical reaction, thereby purifying the elements.
| 4. How is fractional crystallization used in the isolation of elements? |  |
Ans. Fractional crystallization is a method used in the isolation of elements to separate impurities from a mixture. It takes advantage of the differences in solubility between the desired compound and impurities. By gradually cooling down the mixture, the desired compound forms crystals, while the impurities remain in the solution. The crystals can then be separated by filtration, resulting in the isolation and purification of the element.
| 5. What is the role of reduction in the isolation of elements? |  |
Ans. Reduction is a chemical process that plays a significant role in the isolation of elements. It involves the removal of oxygen or the addition of hydrogen to a compound to obtain the pure element. Reduction is commonly used for the extraction of metals from their ores. The process can be carried out through various methods, including the use of carbon, hydrogen, or electrolysis.
Related Searches

















