Organometallic Reagents: Grignard, Gilman and Organocopper | Organic Chemistry PDF Download
Grignard reagents are extremely useful organometallic compounds in the field of organic chemistry. They exhibit strong nucleophilic qualities and also have the ability to form new carbon-carbon bonds. Therefore, they display qualities that are also exhibited by organolithium reagents and the two reagents are considered similar. When the alkyl group attached to a Grignard reagent is replaced by an amido group, the resulting compound is called a Hauser base. These compounds are even more nucleophilic that their Grignard counterparts. Grignard reagents are known for their ability to quickly target carbonyls at their carbon level. Grignard reagents do not, however, function in the presence of protic solvents. Instead of reacting with the desired molecule, the Grignard is so unstable that it can readily accept a proton from a protic solvent. The Grignard then is inert and there is no reaction to the desired molecule.
What are Grignard Reagents?
A Grignard reagent is an organomagnesium compound which can be described by the chemical formula ‘R-Mg-X’ where R refers to an alkyl or aryl group and X refers to a halogen.
They are generally produced by reacting an aryl halide or an alkyl halide with magnesium.
These reagents were discovered by the French chemist Victor Grignard, who won the Nobel Prize in Chemistry in the year 1912 for his work on these compounds.
Grignard Reagents
Grignard reagents (RMgX) are commonly used for organic synthesis. However, these highly reactive compounds are supplied in flammable solvents, which cause extra complexity in their transport. Herein we note that Grignard reagents with linear alkyl chains can be trapped and stabilized by the macrocyclic host pillar arene while retaining their reactivity.
Reactions that form carbon-carbon bonds are among the most beneficial to the synthetic organic chemist. In 1912, Victor Grignard was awarded the Nobel Prize in Chemistry for his discovery of a new sequence of reactions resulting in the creation of a carbon-carbon bond. Grignard synthesis involves the preparation of an organomagnesium reagent through the reaction of an alkyl bromide with magnesium metal.
The Grignard reaction is an organic reaction used to produce a variety of products through the reaction of an organomagnesium compound, also known as an electrophilic “Grignard reagent,” followed by an acidic reaction. The Grignard reagent is formed by the reaction of an alkyl or aryl halide with magnesium metal via a radical mechanism.
Preparation of Grignard Reagents
The process of preparing Grignard reagents is described in the points provided below. It can be noted that many of these reagents can also be purchased commercially.
- These reagents are prepared via the treatment of magnesium with organic halides such as alkyl or aryl halides.
- This is done with the help of solvents comprising ethers (which are described by the formula R-O-R’) because the ligands provided by these solvents help in the stabilization of the organomagnesium compound.
- Water and air are very harmful to this synthesis and can quickly destroy the Grignard reagent which is being formed via protonolysis or via oxidation of the reagent. Therefore, the process must be carried out in air-free conditions.
- Alternatively, the magnesium can be activated to make it consume water when wet solvents are used with the help of ultrasound.
- After the slow induction period of the reaction, the process can be quite exothermic. This is a very important factor to consider while industrially producing the Grignard reagent.
- The organic halides used in these reactions include aryl or alkyl chlorides, bromides, and iodides. Aryl fluorides and alkyl fluorides are not very reactive and are hence not commonly used. However, with the help of Rieke metals, the magnesium can be activated to make the fluoride more reactive.
An illustration detailing the preparation of these reagents is provided below.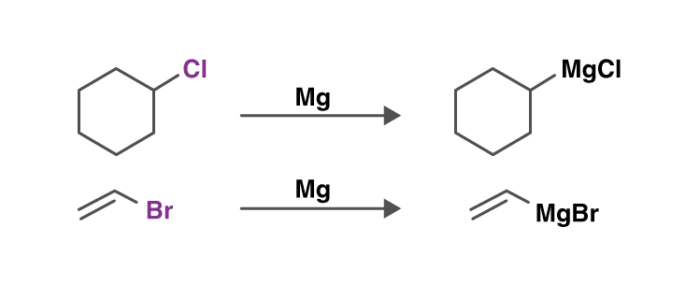
The quality testing of the synthesized Grignard reagents is done via titrations involving protic reagents that do not contain water (since these reagents are highly sensitive to oxygen and water) and a colour indicator. One suitable compound for these titrations is methanol.
Reactions of Grignard Reagents
During a reaction involving Grignard reagents, it is necessary to ensure that no water is present which would otherwise cause the reagent to decompose rapidly. Therefore, the majority of Grignard reactions occur in solvents such as anhydrous diethyl ether or tetrahydrofuran because the oxygen in these solvents stabilizes the magnesium reagent. Grignard reagents are very important reagents in organic chemistry since they can be reacted with a wide range of compounds to form different products. Some of these reactions of these reagents are listed below.
1. Reactions with Carbonyl Group These reagents form various products when reacted with different carbonyl compounds. The most common reaction of Grignard reagents is the alkylation of ketones and aldehydes with the help of R-Mg-X.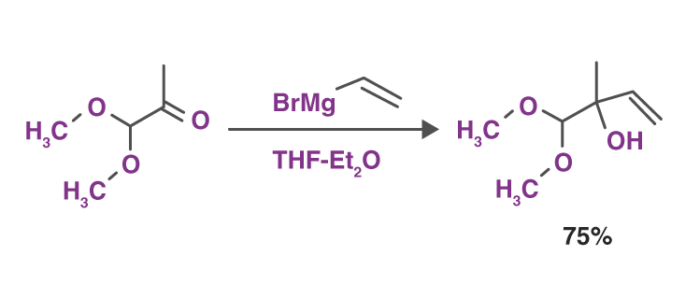
This reaction depicted above is also referred to as the Grignard reaction. The solvents that are used in this reaction include tetrahydrofuran and diethyl ether.
2. Reactions with Non-Carbon Electrophiles
For the formation of new carbon-heteroatom bonds, Grignard reagents and some organolithium compounds are very useful. These reagents can also undergo a transmetallation reaction with cadmium chloride, yielding dialkyl cadmium. This reaction can be written as follows.
2R-Mg-X + CdCl2 → R2Cd + 2Mg(X)Cl
Alkyl chains can be attached to many metals and metalloids with the help of these reagents.
3. Reactions with Organic Halides
Typically, these reagents are quite unreactive towards organic halides which highly contrasts their behaviour towards other halides. However, carbon-carbon coupling reactions occur with Grignard reagents acting as a reactant when a metal catalyst is introduced.
An example of such a coupling reaction is the reaction between methyl p-chlorobenzoate and nonyl magnesium bromide which yields the compound p-nonyl benzoic acid in the presence of the catalyst – Tris(acetylaceto) iron(III).
4. Industrial Reactions
For the production of Tamoxifen, a type of medication used to prevent and treat breast cancer, the Grignard reagent is a vital part of the non-stereoselective process.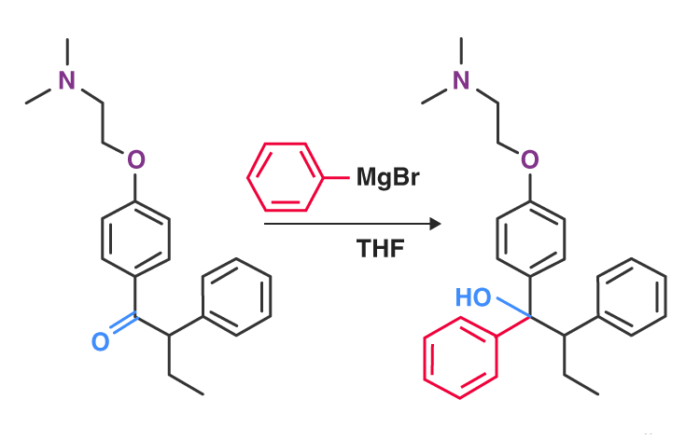
An illustration detailing the Grignard reaction that takes place in the manufacturing process of tamoxifen can be found above.
What are Gilman Reagents?
A Gilman Reagent is an organometallic reagent containing two R-groups (alkyl or aryl), copper, and lithium. The general formula of Gilman reagents can be expressed as R2CuLi. Unlike Grignard reagents, these compounds tend to replace the halide group with an R group when reacted with organic halides. Therefore, Gilman reagents have crucial applications in the Corey-House reaction. Furthermore, relatively complex products can be synthesized from simple blocks via displacement reactions involving the Gilman reagent.
Gilman reagents were discovered by (and are named after) the American organic chemist Henry Gilman. The most common example of a Gilman reagent is lithium dimethylcuprate. When dissolved in diethyl ether, this compound exists as a dimer by forming an 8-membered ring with another lithium dimethylcuprate molecule.
Preparation
Lithium dimethylcopper can be prepared by reacting methyllithium (one of the simplest organolithium reagents with the chemical formula CH3Li) with copper (I) iodide (also known as cuprous iodide, has a chemical formula of CuI) in a reaction environment provided by tetrahydrofuran. The optimum reaction temperature for the preparation of this compound is approximately -78 degrees Celsius.
Reactions Undergone by Gilman Reagents
Gilman reagents act as methylating agents when subjected to conjugate addition reactions with alkynes. The negative charge on the reagent becomes trapped with the ester group via a nucleophilic acyl substitution reaction, resulting in the formation of a cyclic enone. On conjugated enones, these compounds tend to perform 1,4 additions rather than 1,2 additions.
Applications of Gilman Reagents
Some important applications of Gilman reagents are provided below.
- Gilman reagents can be employed to perform conjugate additions on alpha, beta-unsaturated ketones. Grignard reagents are not viable here since they tend to add to the carbonyl carbon.
- Gilman reagents also prove to be highly effective nucleophile in SN2 reactions.
Reactions of organocopper reagents
Reactions of organocopper reagents involve species containing copper-carbon bonds acting as nucleophiles in the presence of organic electrophiles. Organocopper reagents are now commonly used in organic synthesis as mild, selective nucleophiles for substitution and conjugate addition reactions. Since the discovery that copper(I) halides catalyze the conjugate addition of Grignard reagents in 1941, organocopper reagents have emerged as weakly basic, nucleophilic reagents for substitution and addition reactions. The constitution of organocopper compounds depends on their method of preparation and the various kinds of organocopper reagents exhibit different reactivity profiles. As a result, the scope of reactions involving organocopper reagents is extremely broad.
- Organocopper complexes (RCu) are produced when a copper(I) halide and organolithium are combined. In conjunction with Lewis acidic additives such as boron trifluoride etherate, these reagents are used for conjugate addition reactions.
- Lower-order cuprates (R2CuLi, also known as Gilman reagents) result when organocopper complexes are treated with an equivalent of organolithium. Alternatively, they may be formed by the treatment of a copper(I) halide with two equivalents of organolithium. They undergo substitution, conjugate addition, and carbocupration reactions in the presence of the appropriate organic substrates. Mixed Gilman reagents consist of two different R groups, one of which is typically a non-transferable "dummy" group.
- Lower-order cyanocuprates (RCu(CN)Li) are similarly derived from an organolithium compound and copper(I) cyanide; however, intermediate organocopper complexes do not form during this reaction and thus only a single equivalent of organolithium reagent is necessary. Cyanocuprates undergo SN2' substitution in the presence of allyl electrophiles and conjugate addition reactions in the presence of enones.
- Higher-order cyanocuprates (R2Cu(CN)Li2) are formed upon the reaction of two equivalents of organolithium with copper(I) cyanide. These reagents are more reactive towards substitution than the corresponding lower-order cyanocuprates.
Mechanism and Stereochemistry
Substitution Reactions
The mechanism of nucleophilic substitution by lower-order organocuprates depends in a profound way on the structure of the substrate, organocuprate, and reaction conditions. Early evidence suggested that a direct SN2 displacement was occurring; however more recent results suggest that invertive oxidative addition of copper(I) into the carbon-leaving group bond takes place, generating a copper(III) intermediate which then undergoes reductive elimination to generate the coupled product. Both of these mechanisms predict inversion at the electrophilic carbon, which is observed in a number of cases. On the other hand, experiments with radical traps and the observation of racemization during substitution suggest a radical mechanism.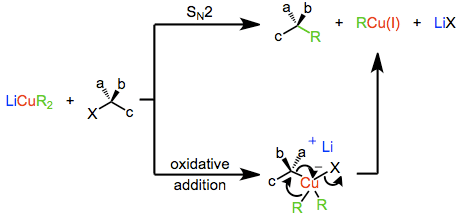
Conjugate Addition Reactions
In 1941, Kharash discovered that Grignard reagents add to cyclohexenone in presence of Cu(I) resulting in 1,4-addition instead of 1,2-addition. This work foreshadowed extensive studies on the conjugate additions to enones with organocuprates. Note that if a Grignard reagent (such as RMgBr) is used, the reaction with an enone would instead proceed through a 1,2-addition. The 1,4-addition mechanism of cuprates to enones goes through the nucleophilic addition of the Cu(I) species at the beta-carbon of the alkene to form a Cu(III) intermediate, followed by reductive elimination of Cu(I). In the original paper describing this reaction, methylmagnesium bromide is reacted with isophorone with and without 1 mole percent of added copper(I) chloride (see figure).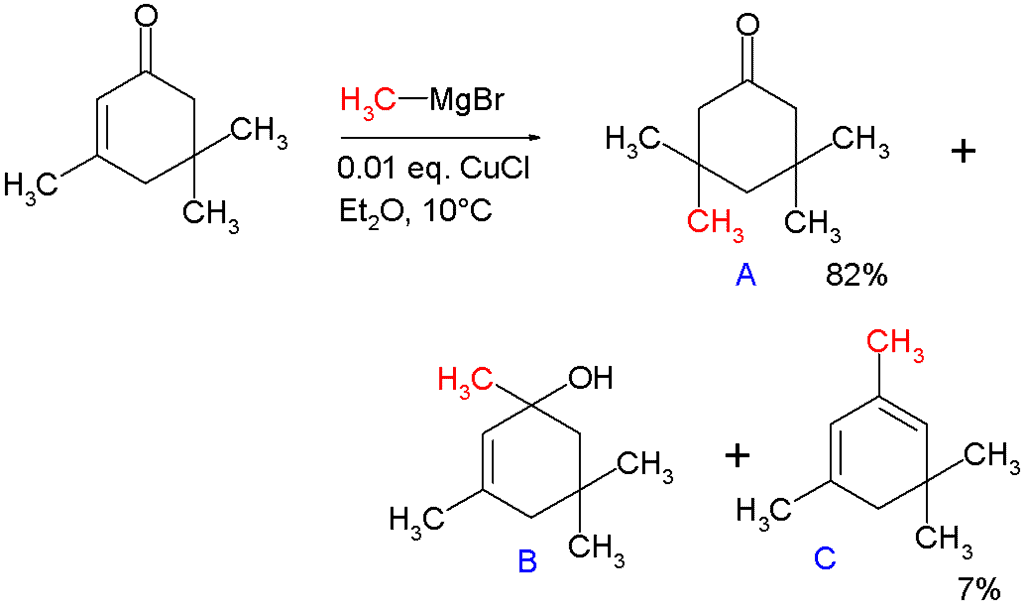
Without added salt the main products are alcohol B (42%) from nucleophilic addition to the carbonyl group and diene C (48%) as its dehydration reaction product. With added salt the main product is 1,4-adduct A (82%) with some C (7%).
A 1,6-addition is also possible, for example in one step of the commercial-scale production of fulvestrant: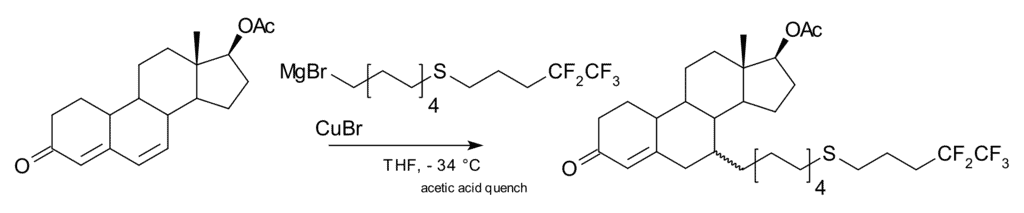
Enantioselective Variants
Diastereoselective conjugate addition reactions of chiral organocuprates provide β-functionalized ketones in high yield and diastereoselectivity. A disadvantage of these reactions is the requirement of a full equivalent of enantiopure starting material.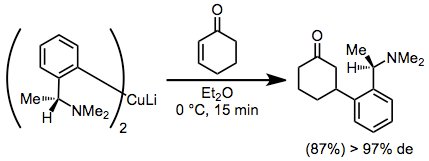
More recently, catalytic enantioselective methods have been developed based on the copper(I)-catalyzed conjugate addition of Grignard reactions to enones. The proposed mechanism involves transmetalation from the Grignard reagent to copper, conjugate addition, and rate-determining reductive elimination (see the analogous upper pathway in equation (2)).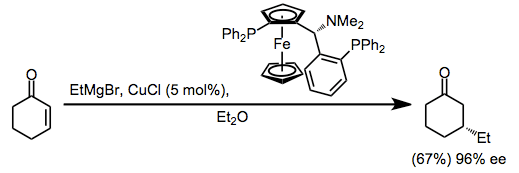
Catalytic reactions
Vinyl and aryl Grignard reagents couple with primary alkyl halides in the presence of a catalytic amount of a copper(I) halide salt. The use of Li2CuCl4 rather than simple copper(I) halide salts (CuX) improves yields of these coupling reactions.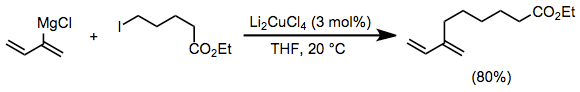
The addition of Grignard reagents to alkynes is facilitated by a catalytic amount of copper halide. Transmetalation to copper and carbocupration are followed by transmetalation of the product alkene back to magnesium. The addition is syn unless a coordinating group is nearby in the substrate, in which case the addition becomes anti and yields improve.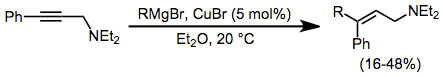
Stoichiometric reactions
Propargyl methanesulfinates are useful substrates for the synthesis of allenes from stoichiometric organocopper complexes. In this case, the complexes were generated in situ through the combination of a Grignard reagent, copper(I) bromide, and lithium bromide. Organocopper complexes very often need Lewis acid activation in order to react efficiently; magnesium bromide generated in situ serves as an activating Lewis acid in this case.
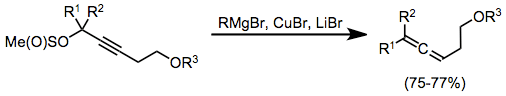
Alkenylcopper complexes, easily generated through carbocupration, are useful for the introduction of a vinyl group in the β position of a carbonyl compound. In this case, as above, magnesium bromide is serving as an activating Lewis acid.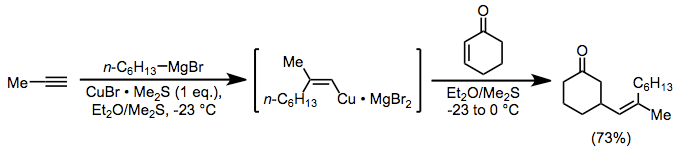
Epoxide opening with organocuprates is highly selective for the less hindered position. Substitution takes place with complete inversion of configuration at the electrophilic carbon.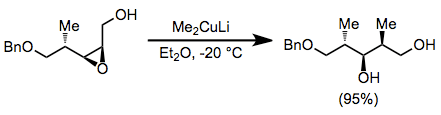
Generally, organocuprates react with allylic electrophiles in an anti SN2 fashion. In the reaction below, nearly complete inversion of configuration was observed despite the presence of a second stereocenter in the ring.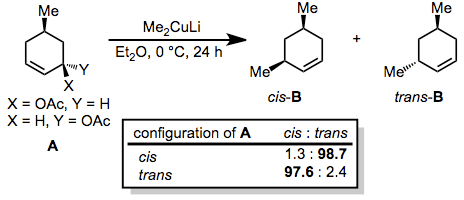
Conjugate addition of organocuprates is widely used in organic synthesis. Vinyl ether cuprates serve as convenient acyl anion equivalents in conjugate addition reactions to enones. The resulting enol ethers can be hydrolyzed to 1,4-diketones, which are difficult to access using conventional carbonyl chemistry.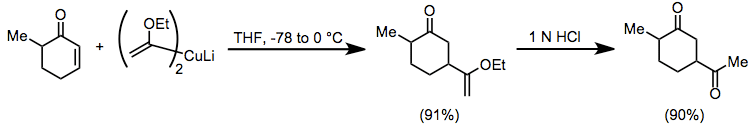
The use of additives in conjunction with a stoichiometric amount of organocopper complexes enhances the rate and yield of many reactions. Organocopper complexes in particular react sluggishly in the absence of a Lewis acid. Although magnesium bromide generated in situ from the reaction of Grignard reagents and copper(I) halides can serve this role (see above), external Lewis acids are also useful. In the presence of boron trifluoride etherate, organocopper complexes are able to add to sterically congested enones in moderate yield (effecting the same transformation with an organocuprate would be difficult).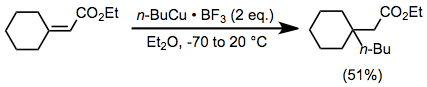
Boron trifluoride etherate is also useful as an additive in reactions of higher-order cyanocuprates. The use of the 2-thienyl group as a "dummy" substituent in the cyanocuprate conserves the potentially valuable organolithium reagent used to generate the cyanocuprate (as only the dummy group is present in copper-containing byproducts). In the absence of boron trifluoride etherate, no reaction was observed in this case.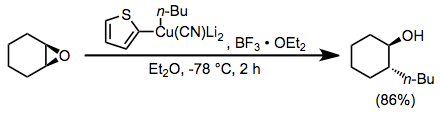
Conjugate addition reactions of higher-order cyanocuprates represent another useful application for boron trifluoride etherate. The vinyl group is transferred selectively in this reaction; this is in contrast to substitution reactions employing the same reagent, which result in selective transfer of the methyl group.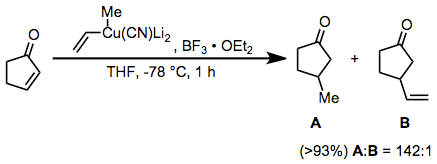
Alkylation of amines
Secondary amines can be alkylated with cuprates. The reaction is based on the oxidative coupling of lithium alkyl copper amide which is reported to form in situ during the reaction between lithium dialkylcuprates and primary or secondary amides.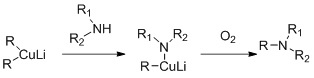
Synthetic Applications
ecause the stereoselectivity of carbocupration is extremely high, the reaction has been applied to the synthesis of pheromones in which the geometric purity of double bonds is critical. One example is the insect pheromone of Cossus cossus, which is synthesized by syn-selective carbocupration of acetylene and alkylation of the resulting organocuprate in the presence of added phosphite.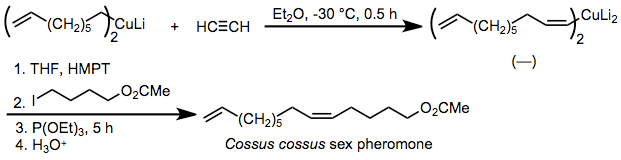
|
44 videos|102 docs|52 tests
|
FAQs on Organometallic Reagents: Grignard, Gilman and Organocopper - Organic Chemistry
| 1. What are Grignard reagents? |  |
| 2. What are Gilman reagents? |  |
| 3. What are some reactions of organocopper reagents? |  |
| 4. How are Grignard reagents prepared? |  |
| 5. What are some applications of Grignard and Gilman reagents in organic synthesis? |  |





















