Solids, Liquids and Gases - 1 Class 4 Worksheet Science
Q1: Multiple Choice Questions (MCQs).
(i) The mixture of salt and water is called
(a) solute
(b) solvent
(c) solution
(d) matter
Ans: (c)
The solute is the substance which is dissolved by the solvent. For example, in a solution of salt and water, water is the solvent and salt is the solute. Solutions are formed because the molecules of the solute are attracted to the molecules of the solvent.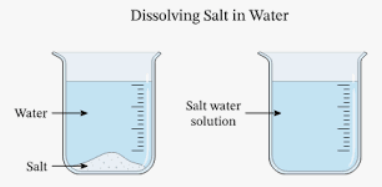
(ii) Which of these has a fixed shape and volume?
(a) Solid
(b) Liquid
(c) Gas
(d) All of these
Ans: (a) Solids have fixed shape and volume.
Solids have fixed shape and volume.
(iii) Which of the following is not always true for matter?
(a) Has weight
(b) Takes up space
(c) Can be seen
(d) Is made up of molecules
Ans: (c)
Any material can be seen if the molecules are dense or large but if the molecules are scattered like in gaseous state one cannot see it so every matter can be seen as a false statement.
(iv) The distance between the molecules of which of these is the highest?
(a) Solid
(b) Liquid
(c) Gas
(d) Same in all of these
Ans: (c)
The distance between the molecules of Gas is high compared with liquid and solid.
Q2: True & False.
(i) Ice cube melts into water in freezing.
Ans: False
This statement is incorrect. Freezing is the process by which a liquid (in this case, water) changes into a solid (ice) when its temperature drops below its freezing point. Conversely, when ice melts, it changes from a solid state to a liquid state as it warms up, typically at temperatures above its melting point.
(ii) Insoluble substances can be seperated from liquid by sedimentation only.
Ans: False
Sedimentation alone is not always sufficient to separate insoluble substances from a liquid. Sedimentation is the process by which solid particles in a liquid settle down over time due to gravity. However, to completely separate insoluble substances, additional processes like filtration or decantation may be required, depending on the specific properties of the substances and the desired outcome.
(iii) Both solids and liquids have definite shapes.
Ans: False
This statement is inaccurate. Solids have definite shapes, meaning they maintain their shape and volume under normal conditions. Liquids, on the other hand, do not have a definite shape; they take the shape of the container they are in while maintaining a fixed volume. Gases, in contrast, neither have a definite shape nor a definite volume, as they can expand to fill the space available.
(iv) In evaporation process, a liquid changes into solid.
Ans: False
This statement is incorrect. In the process of evaporation, a liquid changes into a gas, not a solid. Evaporation occurs when a liquid, such as water, absorbs heat energy from its surroundings and its molecules gain enough kinetic energy to break free from the liquid phase and enter the gas phase. This is why, for example, water left in an open container will slowly evaporate into the air over time, but it won't turn into a solid.
Q3: Fill in the blanks.
(i) Intermolecular space is least in case of _____.Ans: Solids
In solids, particles (atoms, molecules, or ions) are closely packed and have very little intermolecular space. The strong forces of attraction between particles in solids hold them in a fixed position, resulting in a definite shape and volume. This close packing and minimal intermolecular space are what characterize the solid state of matter.
(ii) Liquids do not have definite_____.
Ans: Shape
Unlike solids, which have a definite shape, liquids do not have a fixed shape. Liquids take the shape of the container they are in due to the relatively weak forces of attraction between their particles. However, they do have a definite volume because the particles are still close together.
(iii) Sand is _____ in water.
Ans: Insoluble
When we say that sand is insoluble in water, it means that sand does not readily dissolve in water. Sand consists of larger solid particles, and when mixed with water, it does not undergo a chemical reaction to form a solution. Instead, sand particles tend to settle down in water due to their insolubility, a process called sedimentation. This makes it possible to separate sand from water by allowing it to settle and then pouring off the water.
Q4: Match the following.

Ans: 1. Change of solid state into liquid - Melting
The process of a solid becoming a liquid is called melting (an older term that you may see sometimes is fusion).
2. Sugar in lemonade - solute
In lemonade, water is called the solvent. Sugar and lemon juice are solutes. A solute is matter that dissolves in a solvent. The solvent is often a liquid, such as water.
3. Change of liquid state into solid - Freezing
Freezing occurs when a liquid is cooled and turns to a solid. Eventually the particles in a liquid stop moving about and settle into a stable arrangement, forming a solid.
4. Change of vapour into liquid - condensation
Condensation of a vapour to form a liquid or a solid is the reverse of vaporization, and in the process heat must be transferred from the condensing vapour to the surroundings.
5. Conversation of liquid into vapour - Evaporation
Vaporization, conversion of a substance from the liquid or solid phase into the gaseous (vapour) phase. If conditions allow the formation of vapour bubbles within a liquid, the vaporization process is called boiling.
Q5: Answer the following questions in brief.
(i) What do you understand by the term freezing?
Ans: Freezing is a phase transition where a liquid turns into a solid when its temperature is lowered below its freezing point. In accordance with the internationally established definition, freezing means the solidification phase change of a liquid or the liquid content of a substance, usually due to cooling.
(ii) What is matter?
Ans: Matter is anything that has weight and takes up space. All the living things such as birds, animals and plants, and all the non-living things such as chairs, tables, balls, air and water are matter. All these things have their own weight and take up space.
(iii) What is condensation?
Ans: Condensation is the process by which water vapor (water in its gas form) turns into liquid. It happens when molecules of water vapor cool and collect together as liquid water. Water vapor can be found on the outside of cold glasses, the warm side of windows, and in the clouds up in the air.
(iv) Define the following:
(a) Solution
Ans: A solution is a homogeneous mixture of two or more components in which the particle size is smaller than 1 nm. Common examples of solutions are the sugar in water and salt in water solutions, soda water, etc.
(b) Solvent
Ans: A solvent is a molecule that has the ability to dissolve other molecules, known as solutes. A solvent can be solid, liquid or gas. The molecules of the solvent work to put the solute molecules apart.
(c) Solute
Ans: A substance that is dissolved in a solution is called a solute. In fluid solutions, the amount of solvent present is greater than the amount of solute. One best example of solute in our day to day activity is salt and water.
(v) Write any two properties of solids.
Ans:
- A solid has a definite shape and volume.
- Solids in general have higher density.
- In solids, intermolecular forces are strong.
- Diffusion of a solid into another solid is extremely slow.
|
50 videos|90 docs|28 tests
|
FAQs on Solids, Liquids and Gases - 1 Class 4 Worksheet Science
| 1. What are the three states of matter? |  |
| 2. How do solids, liquids, and gases differ from each other? |  |
| 3. Can a substance change from one state of matter to another? |  |
| 4. What causes the changes in state of matter? |  |
| 5. What are some examples of substances in each state of matter? |  |


















