Class 9 Exam > Class 9 Notes > Lab Manual: Density of Solid
Lab Manual: Density of Solid - Class 9 PDF Download
Objective
To determine the density of solid (denser than water) by using a spring balance and a measuring cylinder.
Theory
- Density: The density of a substance is defined as the mass per unit volume,[D = M/V]
Here, D = Density of the body
M = Mass of the body
V = Volume of the body. - S.I. unit of density = Kgm-3 or Kg/m-3
c.g.s. unit of density = g/cm-3 or g cm-3 - Floating bodies: The density of water is 1 g/cm3 (c.g.s. system) and 1000 kg/m3 (S.I. system).
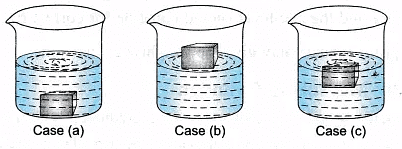
- Case (a) If the density of a body is more than 1 g/cm3 or 1000 kg/m3 then the body will sink in water.
- Case (b) If the density of a body is less than 1 g/cm3 or 1000 kg/m3 then the body will float on water.
- Case (c) If the density of a body is same i.e. 1 g/cm3 or 1000 kg/m3 then the body will half float and half submerge in water.
- The force due to the gravitational attraction of the earth that acts on a body is called weight.
- (Weight) Force = mass x acceleration.
Force = mass x acceleration due to gravity (g)
Force = mass x g
i.e Weight = m x g - Weight of a body = Force on the body.
- S.I. unit = Newton = 1 kg m/s2
- N =1 kgf= 1 kilogram force,
i.e g = 9.8 m/s2 - Weight is measured by spring balance.
Materials Required
Procedure
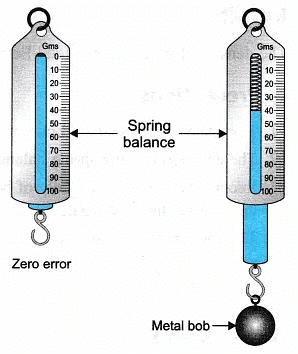
- Tie a metal bob (or any solid) with the string of cotton to the hook of the spring balance. The spring balance should be checked for any error. Let the zero error be ‘x’.
- Hold the spring balance (or tie it to the stand), suspended with the metal bob in air. Measure the weight of the bob. Let its weight be ‘WF‘
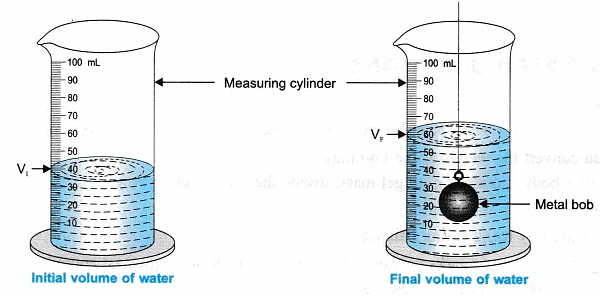
- Pour the water in the measuring cylinder and record the initial volume of water, let it be ‘ V1‘
- Suspend the metal bob into the measuring cylinder with water. The bob should not touch the base, nor the sides of the cylinder.
The water level rises, measure the increased water level, let this volume be ‘ VF‘ - Record all your observations in the observation table and do the calculation to find the density of a given solid metal bob.
Observations
- Weight of the given Metal Bob = 400N
- Mass of the Metal Bob = 400/9.8 = 40.8g

- Volume of water displaced by solid (metal bob) = 20 mL.
- Density of a solid (metal bob) =40.8g/20ml = 2.04 g/cm3
1 mL of water = 1 cm3
Result
Precautions
- The spring balance should be sensitive.
- The zero error in the spring balance should be recorded before it is used to find the weight of solid.
- Record the readings carefully of both spring balance and measuring cylinder by keeping the level of eye and the mark of reading same/parallel.
- The solid/metal bob should not touch the bottom, or sides of the measuring cylinder.
- If the zero error in spring balance is 1 N then subtract this error from the final reading of the weight of solid/ metal bob.
FAQs on Lab Manual: Density of Solid - Class 9
| 1. What is the definition of density? |  |
Ans. Density is a physical property that measures the compactness of a substance. It is defined as the mass of a substance per unit volume. The formula for density is density = mass/volume.
| 2. How is the density of a solid calculated? |  |
Ans. To calculate the density of a solid, you need to measure its mass and volume. First, measure the mass of the solid using a balance. Then, measure the volume of the solid by either direct measurement (e.g., using a ruler for a regular-shaped solid) or by displacement method (e.g., immersing the solid in water and measuring the change in water volume). Finally, divide the mass by the volume to obtain the density.
| 3. Why is density considered an important property of solids? |  |
Ans. Density is an important property of solids because it helps in identifying and characterizing different substances. It can be used to distinguish between materials, determine the purity of substances, or even assess the quality of a material. Moreover, density is also used in various scientific and engineering applications such as designing structures, calculating buoyancy, and understanding the behavior of materials under different conditions.
| 4. How does the density of a solid affect its floating or sinking behavior in a liquid? |  |
Ans. The density of a solid determines whether it will float or sink in a liquid. If the density of the solid is lower than the density of the liquid, it will float. This is because the buoyant force acting on the solid is greater than its weight, causing it to rise to the surface. Conversely, if the density of the solid is higher than the density of the liquid, it will sink. In this case, the weight of the solid is greater than the buoyant force, causing it to sink to the bottom.
| 5. Can the density of a solid change? |  |
Ans. The density of a solid generally remains constant under normal conditions. However, certain factors such as temperature and pressure can affect the density of a solid. For example, as the temperature of a solid increases, its particles tend to expand, resulting in a decrease in density. Similarly, changes in pressure can also cause variations in density. However, these changes are usually small and may not be noticeable in everyday situations.
Related Searches















