Lab Manual: Properties of Acids & Bases - Class 10 PDF Download
Objective
To study the properties of acids (dil. HCl) and bases (dil. NaOH) by their reaction with- Litmus solution (blue/red)
- Zinc metal
- Solid sodium carbonate
Materials Required
Test tubes, test tube stand, test tube holder, cork, droppers, boiling tube, match-box, burner, flat bottom flask, thistle funnel, beaker, litmus solution/paper (red and blue), glass rod, zinc granules, freshly prepared lime water, solid sodium carbonate and dil. HCl.
Theory
AcidAn acid is a substance which furnishes H+ ions when it is dissolved in water like HCl. Acids turn blue litmus red and do not affect red litmus.
On reacting with zinc metal, HCl forms a salt, zinc chloride (ZnCl2) and hydrogen gas (H2) is liberated.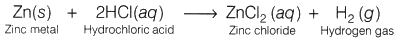 Hydrogen gas burns in air with a blue flame and produces a pop sound.
Hydrogen gas burns in air with a blue flame and produces a pop sound. HCl reacts with sodium carbonate (aqueous/solid) to liberate carbon dioxide (CO2 ) which turns lime water milky due to the formation of calcium carbonate. When excess of CO2 is passed through the solution, the milkiness disappears.
HCl reacts with sodium carbonate (aqueous/solid) to liberate carbon dioxide (CO2 ) which turns lime water milky due to the formation of calcium carbonate. When excess of CO2 is passed through the solution, the milkiness disappears.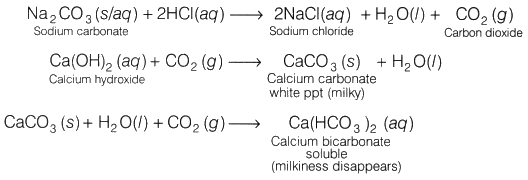
Procedure
1. Litmus Test- Take two test tubes and mark them A and B and put them in a test tube stand. In test tube A take 5 ml of blue litmus solution and in B, take 5mL of red litmus solution (as shown in Fig.1).
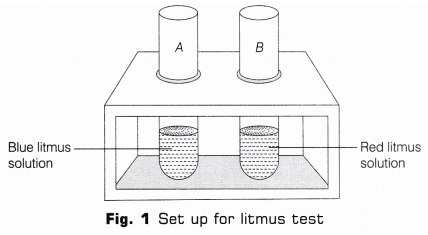
- Using a dropper, add few drops of HCl in each test tube. Stir each tube with separate glass rods B. Note, if there is any colour change occurs in the solutions.
Observation: In test tube A, blue litmus turns red and nothing happens to red litmus. Hence, it can be concluded that acids (like HCl) turns blue litmus to red.
2. Reaction with Zinc (Zn) Metal
- Take few zinc granules in a clean and dry test tube.
- Add HCl to the test tube containing zinc granules, such that zinc granules are totally submerged in the acid.
- Place a cork having glass delivery tube.
- A vigorous reaction will take place after 2-3 minutes, with the evolution of a colourless, odourless gas.
- On bringing a burning match-stick near the mouth of gas tube, the gas burns with a pale blue flame producing pop sound (as shown in Fig. 2).
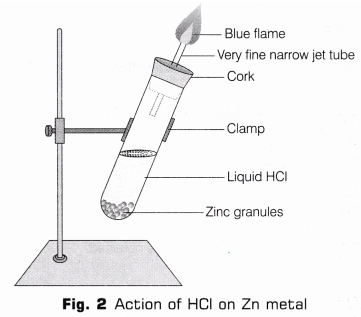 Reaction: 2HCl(aq) + Zn(s) → ZnCl2 (aq) + H2
Reaction: 2HCl(aq) + Zn(s) → ZnCl2 (aq) + H2
Observation: Acids (like HCl) liberate hydrogen (H2) gas on reacting with active metals like zinc (Zn) which burn with a pop sound when burning splinter brought near to it.
3. Reaction with Solid Sodium Carbonate (Na2CO3)
- Take a small amount of solid sodium carbonate in a flat bottom flask and some distilled water in it, shake it well.
- Take a double bore cork with a thistle funnel and a delivery tube fitted in it, fit it on the open end of the flask.
- Now add HCl in the flask through thistle tube.
- Reaction taken place with the evolution of colourless and odourless gas. Then gas is passed through freshly prepared lime water with the help of delivery tube (as shown in Fig. 3).
- The lime water turns milky.
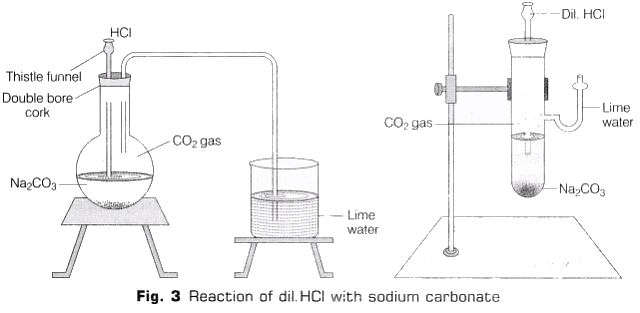 Reaction
Reaction
Na2CO3(aq)+2HCl(aq) → 2NaCl(aq)+ CO2 ↑ + H2O(l)
Ca(OH)2 (aq) + CO2 ↑ → CaCO3(s) + H2O (l)
CaCO3 (s) + H2O(l) + CO2 (g) → Ca(HCO3)2 (aq)
Observation
HCl on reacting with sodium carbonate (Na2CO3) liberates carbon dioxide (CO2) gas which turns lime water milky. On passing the gas in excess of lime water, the milkiness disappears.
Observation Table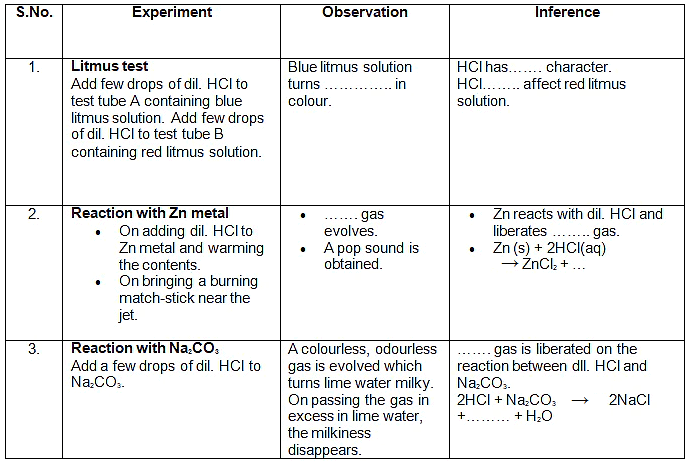
Result
- Hydrochloric acid turns blue litmus solution/paper to red but it does not affect red litmus solution/paper.
- It reacts with zinc metal to liberate hydrogen gas and also forms zinc chloride as a product.
- It reacts with sodium carbonate to liberate carbon dioxide.
Hence, we conclude that hydrochloric acid is acidic in nature.
Precautions
- As HCl is corrosive in nature, it should be handled with care.
- Use small quantities of chemicals.
- Use small quantities of Zn and HCl, otherwise large amounts of H2 will be formed which may cause explosion.
- Use clean zinc metal, otherwise the reaction will occur very slowly.
- Add HCI to Na2CO3, when the apparatus is airtight.
- Observe the milkiness in the lime water soon.
- In case you allow carbon dioxide to pass for a long time through lime water, the milkiness may be removed due to the formation of soluble calcium bicarbonate as depicted in the reaction as follows:
CaCO3(s)+ CO2(g)+ H2O(l) → Ca(HCO3)2(aq). - Shake the solutions and reaction mixtures carefully without spilling.
- Care must be taken while performing combustion test with H2.
FAQs on Lab Manual: Properties of Acids & Bases - Class 10
| 1. What are the properties of acids? |  |
| 2. What are the properties of bases? |  |
| 3. How do acids and bases neutralize each other? |  |
| 4. What are some examples of common acids and bases? |  |
| 5. How can the pH of a substance be determined? |  |



















