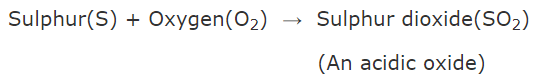Materials: Metals and Non-Metals Class 8 Worksheet Science
Q.1. Which is more reactive iron or copper?
Iron
Q.2. What is ductility?
The property of metal by which it can be drawn into wires is called ductility
Q.3. Why wires are made of aluminum and copper and not of carbon and sulphur?
Aluminum and copper are metals and can be drawn into wires. But carbon and sulphur are non-metals and hence cannot be drawn into thin wires.
Q.4. Which of the two is malleable, phosphorus or iron?
Iron
Q.5. What is the nature of the oxide formed by sulphur?
It is acidic in nature. Generally, oxides of non-metals are acidic in nature.
Q.6. Why there is difference in sound on dropping a metal coin and a piece of coal?
Metals are sonorous i.e. they produce ringing sound when struck hard whereas non-metals non-sonorous. Hence, on dropping a metal coin, ringing sound is produced but no such sound is produced in case of coal.
Q.7. The shape of the iron nail and the aluminium wire changes on beating. Which property of metals is shown by this?
Property of malleability
Q.8. Name the metal which is found in liquid state at room temperature.
Mercury is the only metal which is found in liquid state at room temperature.
Q.9. “The atom of an element remains unaffected by physical changes in the element.” Explain the statement with the help of example.
The atom of an element remains unaffected by physical changes in the element. For example, an atom of liquid sulphur would be exactly the same as the atom of solid or vapour sulphur.
Q.10. How many naturally occurring elements are there?
There are no more than 92 naturally occurring elements.
Q.11. Explain why aluminium foils are used to wrap food items.
Aluminium foils are used to wrap food items because aluminium metal is malleable. Thus, it can be beaten into thin sheets.
Q.12. Can you store lemon pickle in an aluminium utensil? Explain.
Lemon pickle cannot be stored in aluminium utensils because lemon pickle contains acids, which can react with aluminium (metal) liberating hydrogen gas. This can lead to the spoiling of the pickle.
Q.13. Why non-metals cannot be drawn into wires?
Non-metals are not ductile. Therefore they cannot be drawn into wires.
Q.14. How metals and non-metals react with water?
Some metals react with water to produce metal hydroxides and hydrogen gas. Generally, nonmetals do not react with water.
Q.15. Explain reaction between sulphur and oxygen. What is the nature of its oxide formed?
When sulphur burns in air, it combines with oxygen of air to form sulphur dioxide (which is an acidic oxide).
|
33 videos|58 docs|7 tests
|
FAQs on Materials: Metals and Non-Metals Class 8 Worksheet Science
| 1. What are the properties of metals and non-metals? |  |
| 2. What are some examples of metals and non-metals? |  |
| 3. How do metals and non-metals react with water? |  |
| 4. What are alloys and why are they important? |  |
| 5. How are metals and non-metals used in everyday life? |  |