Lakhmir Singh & Manjit Kaur: Materials Metals And Non-Metals- 3 | Lakhmir Singh & Manjit Kaur Solutions: Class 8 Science PDF Download
Q.61.
(A) Why are metals used for making bells?
Metals are sonorous because they produce a unique sound when something hard strikes their surface. As a result, metals are used in making bells or gongs.
(B) Why is phosphorus kept under water?
Metals react with water to produce hydrogen gas. Phosphorus cannot be kept open in the air because it combines with oxygen so easily that it catches fire automatically. For safety, phosphorus is stored under water in chemical laboratories.
Q.62. Which of the following can be beaten into thin sheets? Why?
(a) Zinc
(b) Phosphorus
(c) Sulphur
(d) Oxygen
Correct Answer is Option (a).
Zinc is metal and it can be beaten into thin sheets. The property of metals which allows metals to be hammered into thin sheets is called Malleability.
Q.63. Match the substances given in column A with their uses given in column B:
Column A
(i) Gold
(ii) Iron
(iii) Aluminium
(iv) Carbon
(v) Copper
(vi) Mercury
Column B
(a) Thermometers
(b) Electric wires
(c) Wrapping food
(d) Jewellery
(e) Machinery
(f) Fuel
(i) Gold – (d) Jewellery
Gold is used to make jewellery. This is because these metals are attractive due to their luster and rarity. These metals do not tarnish or react with air.
(ii) Iron – (e) Machinery
Iron is a very strong metal. It is highly malleable and ductile and hence, can be changed into desired shapes.
(iii) Aluminium – (c) Wrapping food
Aluminium is used to make thin foils for packaging medicines, chocolates and food items. This is because it provides a complete barrier to light, oxygen, moisture and bacteria.
(iv) Carbon – (f) Fuel
Carbon can be used as a fuel in the form of coke and charcoal.
(v) Copper – (b) Electric wires
The property of metals which enables them to be drawn into wires is called Ductility. Due to this property metals can be stretched without breaking and drawn into thin wires. For example, Aluminium and copper are examples of highly ductile metals.
(vi) Mercury – (a) Thermometers
Mercury is used for making thermometers. It is liquid at room temperature and is a very good conductor of heat. Even a slightest change in temperature can be noted by using mercury.
Q.64. Give one use each of the following metals:
(a) Iron
(b) Copper
(c) Aluminium
(d) Zinc
(e) Mercury
(a) Iron is used for making machinery and heavy equipment.
(b) The copper is used in making electric wires because it is a good conductor of electricity.
(c) Aluminium is used to make thin foils for packaging medicines, chocolates and food items. This is because it provides a complete barrier to light, oxygen, moisture and bacteria.
(d) Zinc is widely used in the manufacture of many products such as paints, rubber, cosmetics, pharmaceuticals, plastics, inks, soaps, batteries, textiles and electrical equipment.
(e) Mercury is used for making thermometers. It is liquid at room temperature and is a very good conductor of heat. Even a slightest change in temperature can be noted by using mercury.
Q.65. State one use each of the following non-metals:
(a) Oxygen
(b) Nitrogen
(c) Sulphur
(d) Chlorine
(e) Iodine
(a) Oxygen is the nonmetal which is essential for maintaining life and is inhaled during breathing. Oxygen is brought into our lungs via breathing. It is then transported by red blood cells to the entire body to be used to produce energy.
(b) It can be used to replace air which helps to reduce or eliminate oxidation of materials. It is also used to make fertilizers and explosives.
(c) It is used for making fire crackers.
(d) Chlorine is used in water purification process to make drinking water supply germ-free. Chlorine kills pathogens such as bacteria and viruses by breaking the chemical bonds in their molecules.
(e) Iodine is used to make purple colored solution which is applied on cuts and wounds as an antiseptic. It is used in the treatment and prevention of wound infection.
Long Answer Type Questions
Q.66.
(A) What are metals? Name five metals.
Metals are the elements which lose electrons to form cations and are electropositive in nature. For example, Sodium, potassium, Iron, Zinc and Calcium are metals. Metals are good conductors of heat and electricity. Metals are highly malleable and ductile. The property of metals which allows metals to be hammered into thin sheets is called Malleability. Due this unique property, metals can be flattened into thin sheets by hammering and rolling. The property by which iron metal can be hammered to make objects of different shapes such as axe, spade etc. is called Malleability. As a result of high malleability, iron can be flattened or beaten into thin sheets and desired objects can be made by using it. Sodium, Potassium, Copper, Aluminium, Mercury and sodium are metals.
(B) What are non-metals? Name five non-metals.
Nonmetals are the elements that gain electrons and form negative ions. They are also called electronegative elements. For example, chlorine, sulphur, oxygen, bromine, phosphorus and nitrogen are nonmetals. Nonmetals are not malleable and ductile but they are brittle. Nonmetals are bad conductors of heat and electricity. They have low melting and boiling points and are soft. Sulphur, Oxygen, Nitrogen, Fluorine and Chlorine are nonmetals.
Q.67.
(A) What are metalloids? Name two metalloids.
The elements whose properties are intermediate between the properties of metals and nonmetals are called Metalloids. For example, Boron, Silicon and Germanium are metalloids. Heat and electricity can pass through metalloids but not as easily as in metals. Metalloids are also called semiconductors.
(B) Classify the following elements into metals, nonmetals and metalloids:
Copper, Sulphur, Aluminium, Oxygen, Silicon, Nitrogen, Germanium, Mercury, Chlorine, Sodium.
Metals: Metals are the elements which lose electrons to form cations and are electropositive in nature. For example, Sodium, potassium, Iron, Zinc and Calcium are metals. Metals are good conductors of heat and electricity. Metals are highly malleable and ductile.
Copper, Aluminium, Mercury and sodium are metals.
Nonmetals: Nonmetals are the elements that gain electrons and form negative ions. They are also called electronegative elements. For example, chlorine, sulphur, oxygen, bromine, phosphorus and nitrogen are nonmetals. Nonmetals are not malleable and ductile but they are brittle. Nonmetals are bad conductors of heat and electricity. They have low melting and boiling points and are soft.Sulphur, Oxygen, Nitrogen and Chlorine are nonmetals.
Metalloids: The elements whose properties are intermediate between the properties of metals and nonmetals are called Metalloids. For example, Boron, Silicon and Germanium are metalloids. Heat and electricity can pass through metalloids but not as easily as in metals. Metalloids are also called semiconductors.
Silicon and Germanium are metalloids.
Q.68.
(A) What happens when sulphur dioxide is dissolved in water? Write a word equation for the reaction which takes place.
When sulphur dioxide is dissolved in water, then it forms sulphurous acid (H2SO3).
Explanation: Metals react with oxygen to form metallic oxides. These metallic oxides are basic in nature because they react with water to form bases. On the other hand, non-metals react with oxygen to form non-metallic oxides and these oxides differ from metallic oxides because they are acidic in nature. Also, non-metallic oxides react with water to form acids.
Sulphur is non-metal, and sulphur dioxide (SO2) is acidic in nature. Thus, it reacts with water to form sulphurous acid (H2SO3 or H2O.SO2)
The chemical reaction is: SO2 + H2O → H2SO3
(B) What happens when an iron nail is placed in copper sulphate solution? Write word equation of the reaction involved.
If we put Iron nail into copper sulphate solution, the iron nail will displace copper from copper sulphate solution. The following changes will be observed:
- The colour of copper sulphate changes from blue to green after putting iron nails in it.
- Reddish brown colour is deposited on the iron nails. This reddish brown colour is of copper.
Because (Fe) is more reactive than copper (Cu) and is placed above copper in reactivity series of metals, thus and it changes into iron sulphate and copper.
The chemical reaction is given below: Fe (Iron) + CuSO4 (Copper Sulphate) → FeSO4 (Iron Sulphate) + Cu (Copper)
Q.69.
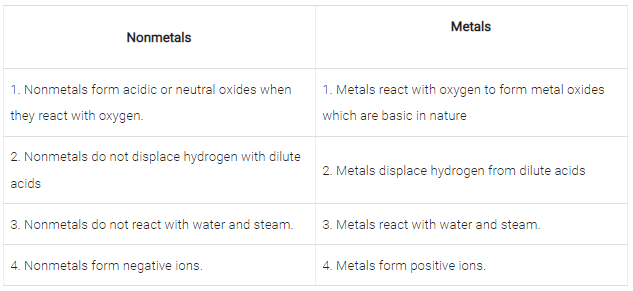
(A) State five characteristics of metals and five characteristics of nonmetals.
Metals are the elements which lose electrons to form cations and are electropositive in nature. For example, Sodium, potassium, Iron, Zinc and Calcium are metals. Following are characteristics of metals:
- Metals are good conductors of heat and electricity.
- Metals are highly malleable and ductile.
- They have very high melting and boiling points.
- Metal oxides are basic in nature.
- Metals displace hydrogen from dilute acids.
Nonmetals: Nonmetals are the elements that gain electrons and form negative ions. They are also called electronegative elements. For example, chlorine, sulphur, oxygen, bromine, phosphorus and nitrogen are nonmetals. Following are the characteristics of nonmetals:
Nonmetals are not malleable and ductile but they are brittle.
- They are bad conductors of heat and electricity.
- They have low melting and boiling points and are soft.
- They form acidic or neutral oxides when they react with oxygen.
- Nonmetals do not displace hydrogen with dilute acids.
(B) State five uses of metals and five uses of non-metals.
The five uses of metals are given below:
- Used for making machinery and heavy equipment. Ex-Iron.
- Used in making electric wires because it is a good conductor of electricity. Ex- copper
- Used to make thin foils for packaging medicines, chocolate, and food items. This is because it provides a complete barrier to light, oxygen, moisture, and bacteria.
- Used in the manufacture of many products such as paints, rubber, cosmetics, pharmaceuticals, plastics, inks, soaps, batteries, textiles and electrical equipment.
- Mercury is a liquid metal and is used for making thermometers. It is liquid at room temperature and is a very good conductor of heat.
The five uses of non-metals are given below:
- Used for making fire crackers.
- Oxygen is an essential non-metal for life. It helps in maintaining life and is inhaled during breathing.
- Chlorine is used in water purification process to make drinking water supply germ-free. Chlorine kills pathogens such as bacteria and viruses by breaking the chemical bonds in their molecules.
- Iodine is a non-metal. It is used to make purple coloured solution which is applied on cuts and wounds as an antiseptic.
- Nitrogen is also a non-metal is used to make fertilizers and explosives.
Q.70. Compare the Chemical Properties of Metals and Non metals in tabular form.
Metals are the elements which lose electrons to form cations and are electropositive in nature. For example, Sodium, potassium, Iron, Zinc and Calcium are metals. Metals are good conductors of heat and electricity. Metals are highly malleable and ductile.
Nonmetals are the elements that gain electrons and form negative ions. They are also called electronegative elements. For example, chlorine, sulphur, oxygen, bromine, phosphorus and nitrogen are nonmetals. Nonmetals are not malleable and ductile but they are brittle. Nonmetals are bad conductors of heat and electricity. They have low melting and boiling points and are soft. Following are the difference in chemical properties between metals and nonmetals.
Multiple Choice Questions (MCQs)
Q.71. An element is soft and can be cut easily with a knife. It is very reactive and cannot be kept open in the air. It reacts vigorously with water. This element is most likely to be:
(a) magnesium
(b) potassium
(c) phosphorous
(d) aluminium
Correct Answer is Option (b)
Potassium reacts with water to potassium hydroxide and hydrogen gas. The reaction potassium with water is highly exothermic in nature. It produces a lot of heat energy and this heat energy can cause the hydrogen gas produced during the reaction, to catch fire. In order to keep it safe potassium is stored in kerosene.
Q.72. Which one of the following four metals would be displaced from the solution of its salt by the other three metals?
(a) zinc
(b) silver
(c) copper
(d) magnesium
Correct Answer is Option (b)
Silver is less reactive than zinc, copper and magnesium. It is below all these metals in the reactivity series of metals.
Q.73. Sulphur element is said to be:
(a) ductile
(b) hard
(c) malleable
(d) brittle
Correct Answer is Option (d)
Sulphur is a nonmetal. It cannot be drawn into wires or changed into sheets. It is very soft and hence, is brittle in nature.
Q.74. An element Z reacts with water to form a solution which turns phenolphthalein indicator pink. The element X is most likely to be:
(a) Sulphur
(b) sodium
(c) carbon
(d) silicon
Correct Answer is Option (b)
Phenolphthalein is an indicator used in acid-base titrations. It turns colorless in acidic solutions and pink in basic solutions. Sodium is a metal which reacts with water to form sodium hydroxide, which is a base. Hence, phenolphthalein turns the solution pink.
Q.75. The non-metal which exists in the liquid state at room temperature is:
(a) fluorine
(b) chlorine
(c) bromine
(d) iodine
Correct Answer is Option (c)
Bromine is the nonmetal which is a red-brown liquid at room temperature. It evaporates readily to form a similarly coloured gas.
Q.76. A basic oxide will be formed by the element:
(a) sulphur
(b) phosphorus
(c) potassium
(d) carbon
Correct Answer is Option (c)
Potassium is a metal. Metallic oxides are basic in nature.
Q.77. “Is malleable and ductile”. This best describes:
(a) a metal
(b) a compound
(c) a non-metal
(d) a mixture
Correct Answer is Option (a)
The property of metals which allows metals to be hammered into thin sheets is called Malleability. Due this unique property, metals can be flattened into thin sheets by hammering and rolling.
Q.78. The metal which will not produce hydrogen gas on reacting with dilute sulphuric acid is:
(a) sodium
(b) silver
(c) iron
(d) zinc
Correct Answer is Option (b)
Silver is least reactive will not produce hydrogen gas with sulphuric acid.
Q.79. The element which is stored under kerosene is:
(a) sulphur
(b) phosphorous
(c) sodium
(d) silicon
Correct Answer is Option (c).
Sodium is stored in kerosene because it is highly reactive and it even reacts with moisture from air and catches fire.
Q.80. Which of the following pairs cannot undergo displacement reaction?
(a) iron sulphate solution and magnesium
(b) zinc sulphate solution and iron
(c) zinc sulphate solution and calcium
(d) silver nitrate solution and copper
Correct Answer is Option (c)
Zinc sulphate solution and calcium cannot undergo displacement reaction.
This is because zinc is more reactive than iron. Hence, iron cannot displace zinc from zinc sulphate solution.
Q.81. Which of the following metal exists in the liquid state at room temperature?
(a) magnesium
(b) manganese
(c) mercury
(d) sodium
Correct Answer is Option (c)
Mercury is used for making thermometers. It is liquid at room temperature and is a very good conductor of heat.
Q.82. The element Z burns in air to form an oxide. The aqueous solution of this oxide turns blue litmus to red. The element Z is most likely to be:
(a) carbon
(b) calcium
(c) iron
(d) magnesium
Correct Answer is Option (a)
Carbon is a nonmetal. Nonmetals form oxides which are acidic in nature. Oxide of carbon is acidic and hence, its aqueous solution will turn blue litmus to red.
Q.83. Which of the following element is a metalloid?
(a) sodium
(b) sulphur
(c) silicon
(d) silver
Correct Answer is Option (c).
Silicon is a metalloid. The elements whose properties are intermediate between the properties of metals and nonmetals are called Metalloids.
Q.84. Which of the following elements will produce an oxide that will dissolve in water to form an acid?
(a) carbon
(b) calcium
(c) chromium
(d) copper
Correct Answer is Option (a)
Carbon is a nonmetal. Nonmetals form oxides which are acidic in nature. Oxide of carbon is acidic.
Q.85. The least reactive metal among the following is:
(a) magnesium
(b) lead
(c) silver
(d) sodium
Correct Answer is Option (c)
Silver is least reactive and is placed below all these metals in reactivity series of metals.
Q.86. You are given a solution of iron sulphate. Which of the following do you think cannot displace iron from iron sulphate?
(a) magnesium
(b) calcium
(c) copper
(d) zinc
Correct Answer is Option (c)
Copper cannot displace iron from iron sulphate solution because copper is less reactive than iron.
Q.87. When a vessel is exposed to moist air for a long time, then a green coating is formed on its surface. The vessel must be made of:
(a) zinc
(b) magnesium
(c) iron
(d) copper
Correct Answer is Option (d)
Copper corrodes by oxidation in which it reacts with oxygen in the air to form copper oxide. Copper oxide then combines with carbon dioxide to make copper carbonate, which gives it a green colour. This process is called corrosion of copper.
Q.88. Which among the following is the most reactive metal?
(a) copper
(b) calcium
(c) iron
(d) magnesium
Correct Answer is Option (b)
Calcium is the most reactive metal than copper, iron and magnesium. It lies above all of these metals in reactivity series.
Q.89. he element whose oxide will turn red litmus solution to blue will be:
(a) sodium
(b) sulphur
(c) carbon
(d) phosphorus
Correct Answer is Option (a)
Metals react with oxygen to form metallic oxides. These metallic oxides are basic because they react with water to form bases. Sodium is a metal, its oxide will be basic in nature and it will turn red litmus solution to blue.
Q.90. Which of the following is not a characteristic property of iron?
(a) malleability
(b) brittleness
(c) ductility
(d) sonorousness
Correct Answer is Option (b)
Brittleness is not a characteristic property of Iron because it is a metal. Malleability, ductility, and sonorousness are the properties of metals.
High Order Thinking Skills Questions
Q.91. Which of the following reactions will not occur? Why not?
(a) Zinc sulphate + Copper ➝ Copper Sulphate + zinc
(b) Copper Sulphate + Iron ➝ iron Sulphate + copper
(a) This reaction will not occur. Zinc is more reactive than copper. Copper will not be able to replace zinc from zinc sulphate solution. A more reactive metal can replace a less reactive metal, but a less reactive one cannot replace a more reactive metal. On the other hand, if we take copper sulphate solution and zinc, then zinc will be able to replace copper from copper sulphate solution. Hence, the above reaction will not take place.
(b) A more reactive metal can replace a less reactive metal, but a less reactive one cannot replace a more reactive metal. This reaction will take place. In this case, Iron is more reactive than copper. In a solution of copper sulphate, iron will react and will be able to displace copper from its solution. In this reaction, iron will change into iron sulphate and copper metal will be produced.
Q.92. One day Reeta went to a jeweller’s shop with her mother. Her mother gave an old gold jewellery to goldsmith to polish. Next day when they brought the jewellery back, they found that there was a slight loss in its weight. Can you suggest a reason for the loss in weight?
When gold jewellery is sent for polishing, it loses weight. While polishing, the outer thin layer of gold which is dull is removed by treatment with a chemical. The chemical treatment of this layer causes a reduction in weight of gold ornaments. This leads to a slight loss in the weight of jewellery.
Q.93. An element burns in air to form an oxide. The aqueous solution of this oxide turns blue litmus paper red. State whether the element is a metal or nonmetal. Name one such element.
The aqueous solution of the oxide turns blue litmus paper red. This means the aqueous solution is acidic in nature. Metals react with oxygen to form metallic oxides. These metallic oxides are basic because they react with water to form bases. On the other hand, nonmetals react with oxygen to form nonmetallic oxides. These oxides are different from metallic oxides because they are acidic in nature. Nonmetallic oxides react with water to form acids. Since, the aqueous solution of this oxide is acidic in nature, the element is a nonmetal. For example, Sulphur is a nonmetal. It will form an oxide called sulphur dioxide which is acidic in nature.
Q.94. An element burns in air to form an oxide. The aqueous solution of this oxide turns turmeric paper red. State whether the element is a metal or nonmetal. Name one such element.
Turmeric is used as a natural indicator. Turmeric is yellow in colour. Turmeric paper turns into red when it is dipped into basic solution. Turmeric paper does not change its colour with acid. The aqueous solution of this oxide turns turmeric paper red. It indicates that the oxide is basic in nature. Metals react with oxygen to form metallic oxides. These metallic oxides are basic because they react with water to form bases. On the other hand, nonmetals react with oxygen to form nonmetallic oxides. These oxides are different from metallic oxides because they are acidic in nature. Nonmetallic oxides react with water to form acids. Hence, the element is a metal. For example, magnesium is a metal, it reacts with oxygen to magnesium oxide, which is basic in nature.
Q.95. The metal X reacts with dilute hydrochloric acid to form a gas Y. The metal X also reacts with sodium hydroxide solution (on heating) to form the same gas Y. When a lighted matchstick is applied, this gas burns by producing a ‘pop’ sound.
(a) name two metals which could behave like X
(b) Name the gas Y
(a) The ‘pop’ sound indicates the presence of hydrogen gas. Metals react with dilute acids to form metallic salts and hydrogen gas. Some metals react with bases to produce hydrogen gas. Generally, non-metals do not react with acids. For example, Aluminium reacts with dilute hydrochloric acid to form Aluminium chloride and hydrogen gas. The balanced chemical equation for the reaction is:
2Al(s) + 6HCl(aq) ➝ 2AlCl3(aq) + 3H2(g).
Aluminium is an amphoteric metal. It reacts with both acids and bases. The reaction is highly exothermic and produces a lot of heat. There is rapid evolution of hydrogen gas during this reaction. Aluminium metal reacts with a base called sodium hydroxide to produce sodium aluminate and hydrogen gas.
(b) The name of the gas is hydrogen.