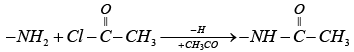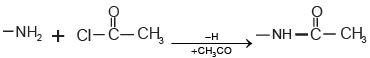Integer Answer Type Questions for JEE: Compounds Containing Nitrogen | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. An organic amino compound reacts with aqueous nitrous acid at low temperature to produce an oily nitroso amine. The compound must have how many minimum carbon atom.
Ans. 2
Q.2. What is the number of benzene derivatives having the molecular formula C7H9N that would respond to carbyl amine test.
Ans. 4
Q.3. The Gabriel synthesis of amines is out lined the following way,
Out of the given amines, how many cannot be prepare by this method.
(1) CH3CH2NH2
(2) CH3NHCH3
(3) CH2 = CH—NH2
(4) 
(5) 
(6) 
(7) 
(8) 
Ans. 5
Only primary amines can be prepared by this method. The second step involves SN2 reaction. Therefore
and CH2 = CH – X are not expected to give SN2.
Q.4. Certain nitrogenous compound with molecular mass (180) show an increase in its molecular mass to 348 after treatment with acetyl chloride. The number of possible – NH2 groups in the molecule is:
Ans. 4
The chemical reaction involved is as follows:
Net increase in mol. Mass on acylation of one –NH2 group
= Mol. Mass of CH3CO group – At. Mass of H
= 43 – 1 = 42
Actual increase in mol. Mass on acylation
= 348 – 180 = 168
no. CH3CO group added = 168/42 = 4
Hence the compound has 4 – NH2 groups.
Q.5. A nitrogenous compound with molecular mass 180 shows an increase in molecular mass to 348 after treatment with acetyl chloride. The number of possible NH2 group in the molecule is.
Ans. 4
Increase in molecular mass on acylation of one -NH2 group is 42. Increase in Molecular mass = 348-180 = 168
No. of -NH2 group = 168/42 = 4
|
446 docs|930 tests
|

|
Explore Courses for JEE exam
|

|