Solved Practice Questions on Ionization Enthalpy | Inorganic Chemistry PDF Download
Q.1. Tick mark the incorrect statement.
(a) The first ionization enthalpy (ΔiH1) of Aluminium is less than that of Magnesium.
(b) The first ionization enthalpy (ΔiH1) of Sodium is less than that of Magnesium.
(c) The second ionization enthalpy (ΔiH2) of Magnesium is greater than the second ionization enthalpy of Sodium.
(d) The third ionization enthalpy (ΔiH2) of Magnesium is greater than the third ionization enthalpy of Aluminium.
Correct Answer is Option (c)
The second I.E. (ΔiH2) of Sodium is greater than the second I.E. of Magnesium. This is because sodium attains the noble gas core after the removal of 1 electron. Thus, the second electron from sodium would be removed from its noble gas core and hence, would require high energy. While in the case of Magnesium, the magnesium receives a noble gas core after the removal of a second electron. Hence, the ΔiH2 of Magnesium is lesser than that of sodium.
Q.2. Pick the correct statement.
(a) The Ionization enthalpy increases along the period.
(b) The Ionization enthalpy decreases along the period.
(c) The electropositive nature of elements increases along the period.
(d) The electronegative nature of elements decreases along the period.
Correct Answer is Option (a)
The Ionization enthalpy depends upon the nuclear charge. As we move from left to right in a period, the nuclear charge and hence, the pull on the outermost electron increases. Hence, the Ionization enthalpy increases as we move from left to right in a period.
Q.3. Which of the following atoms has the lowest first ionization enthalpy?
(a) Potassium
(b) Calcium
(c) Strontium
(d) Rubidium
Correct Answer is Option (d)
Ionization enthalpy is the lowest towards the left within a period. Ionization enthalpy also decreases down the group. Hence, among the given elements, Rubidium has the lowest first ionization enthalpy.
Q.4. The second ionization enthalpy for an element is the required energy for
(a) The removal of a pair of electrons
(b) The removal of 1 mole of electrons from 1 mole of gaseous anions.
(c) The removal of 1 mole of electrons from 1 mole of the monovalent gaseous cations of the given element.
(d) The removal of 2 moles of electrons from a neutral gaseous atom of the element.
Correct Answer is Option (c)
The second ionization enthalpy for an element is the required energy for the removal of 1 mole of electrons from 1 mole of the monovalent gaseous cations of the given element.
Q.5. Which of the following atoms has the highest first ionization enthalpy?
(a) Fluorine
(b) Chlorine
(c) Bromine
(d) Iodine
Correct Answer is Option (a)
As the ionization enthalpy increases towards the right in a period and moving up in a group, Fluorine has the highest first ionization enthalpy.
Q.6. For which of the following electronic configurations, the value of ionization enthalpy is the lowest?
(a) 1s2 2s2 2p6 3s2
(b) 1s2 2s2 2p6 3s1
(c) 1s2 2s2 2p4
(d) 1s2 2s2 2p6
Correct Answer is Option (b)
Since the outermost subshell has only 1 electron. After the removal of 1 electron, the element will acquire a noble gas configuration. Hence, the ionization enthalpy for the electronic configuration (b.) is the lowest.
Q.7. How much energy will be required for the conversion of all of the Magnesium atoms into their respective magnesium cations present in 24 grams of Magnesium vapours? Given that the first (ΔiH1) and second ionization enthalpies (ΔiH2) of magnesium are 737.76 kJ mol-1 and 1450.73 kJ mol-1 respectively.
From the definition of successive ionization enthalpies:
Mg (g) + ΔiH1 → Mg+ (g) + e– (g); ΔiH1 = 737.76 kJ mol-1
Mg+ (g) + ΔiH2 → Mg2+ (g) + e– (g); ΔiH2 = 1450.73 kJ mol-1
Hence, the total energy required to create an Mg2+ ion from Mg (g) is (ΔiH1 + ΔiH2).
∴ total energy required to create an Mg2+ ion = (737.76 + 1450.73) kJ mol-1 = 2188.49 kJ mol-1
Now, 24 g Magnesium contains mole = 1 mol
∴ 0.024 g Magnesium contains mole = 0.024 / 24 mol = 10-3 mol
Hence, the total amount of energy required to convert 10-3 mol of Mg (g) into Mg2+ (g) = 2188.49 kJ mol-1 x 10-3 mol = 2.188 kJ.
Q.8. Why is the first ionization enthalpy of the alkaline earth metals greater than the alkali metals?
This is because the alkaline earth metals have a greater nuclear charge than the alkali metals within the respective period. Hence, the ionization enthalpy increases on moving from left to right within a period.
Q.9. Arrange the following electronic configurations in the increasing order of their ionization enthalpies.
- 1s2 2s2 2p6 3s2,
- 1s2 2s2 2p2,
- 1s2 2s2 2p6 3s1,
- 1s2 2s2 2p6,
- 1s2 2s2 2p3
The ionization enthalpy decreases as the distance of the electron from the nucleus increases. The electronic configuration (a.) and (c.) have their electrons in the third principal energy level. Since, (c.) has only 1 valence electron, so the order of ionization enthalpy in between (a.) and (c.) is (a.) > (c.).
(d.) and (e.) are the completely filled and half-filled electronic configurations. Hence, (d.) is the most stable configuration and has the highest ionization energy. While (e.) has the second highest ionization energy. Hence, the increasing order of their ionization enthalpies is:
(c.) < (a.) < (b.) < (e.) < (d.)
Q.10. What happens if the pressure is increased during the Haber’s process?
On increasing the pressure, the equilibrium of the reaction moves to its right. Hence, the yield of ammonia will increase. However, to apply high pressures, heavy and large equipments are required which are very expensive. Hence, pressure is set at a compromisable unit of 200 atm.
Q.11. Given the first and second ionization potentials of helium (He) are 24.58 eV and 54.4 eV respectively. Calculate the energy (kJ) required to produce one mole of He2+ ions.
The total potential required to make an ion of H2+ = 24.58 eV + 54.4 eV = 78.98 eV
Hence, the total potential required to make 1 mole of H2+ ions = 1 x 78.98 eV = 78.98 eV
As 1 eV = 96.49 kJ mol-1
Hence, 78.98 eV = 78.98 x 96.49 kJ mol-1 = 7620.78 kJ mol-1
∴ The energy (kJ) required to produce one mole of He2+ ions is 7620.78 kJ.
Q.12. The ΔiH1 of carbon is greater than the ΔiH1 of boron, while the ΔiH2 of boron is greater than that of carbon. Explain.
Since both carbon and boron belong to the 2nd period, their electronic configurations must be compared to get an explanation.
C = 1s2 2s2 2p2 B = 1s2 2s2 2p1
Carbon and Boron have their outermost electron in the same subshell i.e. 2p. Carbon has comparatively higher nuclear charge and hence more nuclear pull on the outermost electron than in boron. Hence, ΔiH1 of boron is less than that of carbon.
After the removal of an electron from carbon and boron, the electronic configuration:
C+ = 1s2 2s2 2p1 B+ = 1s2 2s2
Since, the monovalent Carbon and Boron have their outermost electron in 2p and 2s subshell respectively. The s-subshells are more penetrated towards the nucleus, hence have more effect of nuclear attraction on its electrons. Hence, ΔiH2 of Boron is higher than carbon.
Q.13. Out of Fluorine and Chlorine, which one has the lowest first ionization enthalpy?
Chlorine has the lower ΔiH1 owing to its larger size and stronger shielding effect.
Q.14. Arrange the following ions in decreasing order of ionic radii: Li2+, He+, and Be3+.
Li2+, He+, and Be3+ are isoelectronic ions. The number of protons present in Li2+, He+, and Be3+ are 3, 2 and 4 respectively. Hence, the decreasing order of ionic radii is He+ > Li2+ > Be3+.
Q.15. Which among the following have the largest and the smallest sizes?
Mg2+, Mg, Al3+, Al
Both Mg and Al belong to the third period. As the atomic radii decreases on moving from left to right in a period due to increasing nuclear charge. Hence, Mg has a larger atomic radius than Al.
Now, as the cations are smaller in size than their parent atoms, Mg2+ is smaller than Mg and Al3+ is smaller than Al. As Mg2+ and Al3+ are isoelectronic, Al3+ is smaller than Mg2+.
Hence, Mg has the largest and Al3+ has the smallest size.
Q.16. Out of Na, Mg, Si, and P, which one has the greatest difference in between their ΔiH1 and ΔiH2?
Na being an alkali metal has the lowest value of ΔiH1. However, after the removal of an electron, Na acquires a noble gas core, hence, the ΔiH2 of Na is very high. Hence, the difference between the ΔiH1 and ΔiH2 of Na is the highest.
In case of Mg, Si and P, the value of ΔiH1 is higher than Na, but their ΔiH2 is not very high. Hence, the difference between the ΔiH1 and ΔiH2 of these elements is not very high.
Q.17. Explain why cations are smaller and anions are larger in size?
The size of a cation is always smaller than its parent atom. This is because after the removal of 1 or more electrons, the effective nuclear charge experienced by the rest of the electrons increases.
As a result, the force of attraction by the nucleus on the remaining electrons increases and hence, the size of the ion decreases.
While in case of anions, the anionic size is always greater than the parent atom. This is because the addition of 1 or more electrons to the neutral atom decreases the effective nuclear charge, hence, the force of attraction experienced by the electrons decreases. Hence, the anionic size increases.
Q.18. Given are the first and second ionization enthalpies of some elements in kJ mol-1.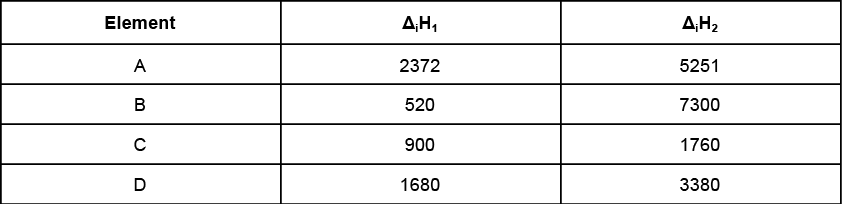
(a) Which of the above is the most reactive metal?
(b) Which of the above is the most reactive non-metal?
(c) Which of the above is a noble gas?
(a) Since the element B has a very less ΔiH1, this implies that the element B is very reactive.
However, the ΔiH2, for B is very high, this implies that B must be a very reactive metal which leaves its second electrons at a very high energy provided. Hence, element B must be a very reactive alkali metal.
(b) The ΔiH1 of the element D is very high but its ΔiH2 is not as considerably high. Hence, the element D must be a reactive non- metal, preferably a halogen.
(c) Element A has the highest value of Δ iH1 but ΔiH2 is not so high. Hence, A must be a noble gas.
Q.19. Would you expect the first ionization enthalpies of the two isotopes of hydrogen to be the same or different?
The ionization energy depends upon various factors including the electronic configuration of the atom and the nuclear charge. The electronic configuration and the nuclear charge (number of protons) of the two isotopes are the same. Hence, the two isotopes must have the same ionization energies.
Q.20. Chlorine can be converted into the chloride ion more easily than the conversion of Fluorine into fluoride ion. Explain.
This is because the electron gain enthalpy of Chlorine is more negative than that of Fluorine.
|
50 videos|92 docs|41 tests
|
FAQs on Solved Practice Questions on Ionization Enthalpy - Inorganic Chemistry
| 1. What is ionization enthalpy? |  |
| 2. How is ionization enthalpy related to the reactivity of elements? |  |
| 3. What factors affect the ionization enthalpy of an atom? |  |
| 4. How does ionization enthalpy vary across a period and down a group in the periodic table? |  |
| 5. How is ionization enthalpy measured? |  |
















