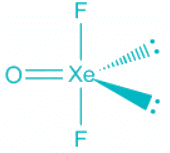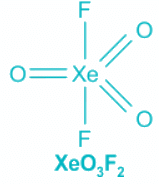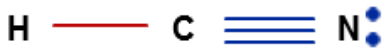Solved Practice Questions on VSEPR Theory and Shapes of Molecules | Inorganic Chemistry PDF Download
Q.1. Solved Practice Questions on VSEPR Theory and shapes of molecules
(a) XeF2
(b) CIF3
(c) IF5
(d) SF4
Correct Answer is Option (a)
Concept
Lone pair-Lone pair repulsion -
- According to VSEPR theory, electron pairs either in chemical bond or lone pair repel each other.
- The extent of repulsion reflects in the overall shape of the molecule.
- Lone pair-lone pair repulsion is the repulsion between the various number of lone pairs present in the molecule.
- Lone pairs are present very close to the nucleus and occupy more space. Hence lone pair-lone pair repulsion is much more than the lone pair-bond pair or bond pair-bond pair repulsion.
- The order of repulsion according to VSEPR theory - Lone pair-lone pair > Lone pair-bond pair > bond pair-bond pair.
- More the no. of lone pairs of electrons in the molecule, greater will be the lone pair - lone pair repulsion.
Explanation:
- Lone pair-lone pair repulsion is the most powerful repulsion depending upon the no. of lone pair present in the molecule.
- Formula to calculate the number of lone pair
- Number of lone pairs on central atom = (Total no. of valence shell electrons on central atom - No. of shared electrons by the central atom) / 2
- The more the number of lone pairs in the molecule more will be the lone-pair lone pair repulsion.
Case 1 : XeF2
- Total no. of valence electrons = 8 (Xe) + 7 (F) + 7(F) = 22 electrons
- Total no. of valence electron on Xe = 8
- no. of valence electron participating in bonding = 2
- Leftover electron = 6
- Total no. of lone pair on Xe = 6 ÷ 2 = 3
Case 2 : ClF3
- Total no. of valence electrons = 7(Cl) + 7(F) + 7(F) + 7(F) = 28 electrons
- Total no. of valence electrons on Cl = 7
- Total no. of shared electrons by Cl = 3
- Total no. of leftover electrons = 7-3 = 4
- Total no. of lone pairs on Cl = 4÷ 2 = 2
Case 3 : IF5
- Total no. of valence electron in molecule = 7(I) + 7(F) + 7(F) + 7(F) + 7(F) + 7(F)
- Total no. of valence electron on I = 7
- total no. of shared electron by I = 5
- leftover electron = 7-5 = 2
- Total no. of lone pairs on I = 2 ÷ 2 = 1
Case 4 : SF4
- total no. of valence electron in the molecule = 6(S) + 7(F) + 7(F) + 7(F) + 7(F) = 34 electrons
- Total no. of valence electron on S = 6
- Total no. of shared electron by S = 4
- Leftover electron = 6-4 = 2
- Total no. of lone pairs on S = 2 ÷ 2 = 1
Conclusion:
Therefore, lone pair-lone pair repulsion is maximum in XeF2 as it is having more no. of lone pairs i.e. 3.
Hence, the correct answer is option 1.
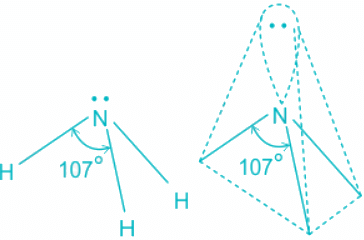
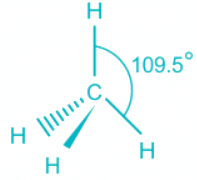
Q.2. What is the bond angle expected for H-N-H is NH3 molecule?
(a) 120°
(b) Over 109° but the less than 120°
(c) 109.5°
(d) Less than 109° but over 90°
Correct Answer is Option (d)
Concept:
- The Valence Shell Electron Pair Repulsion (VSEPR) Theory provides the procedure to determine the shape of the covalent molecule.
- The electron pair that is involved in the bonding is termed as the bond pair, while the one not involved in bonding is termed as the lone pair of electron.
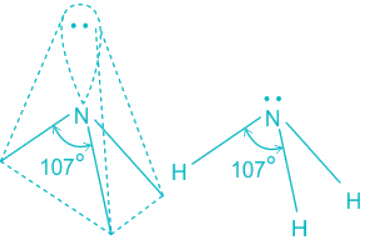
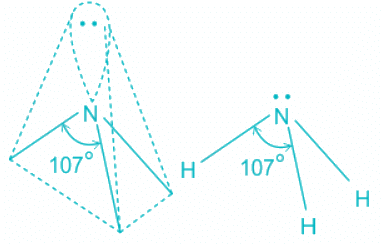
- The ammonia molecule has a trigonal pyramidal shape with the three hydrogen atoms and an unshared pair of electrons attached to the nitrogen atom.
Key Points
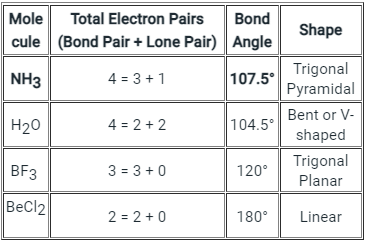
NH3 Bond angles:
- There are three single bonds and one lone pair of electrons in NH3 molecule.
- It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure.
- The shape is distorted because of the lone pairs of electrons.
- This pair exerts repulsive forces on the bonding pairs of electrons.
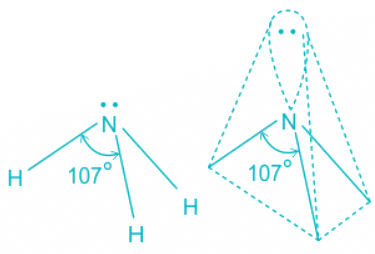
- Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it decreases to 107 degrees due to the lone pair on the nitrogen atom.
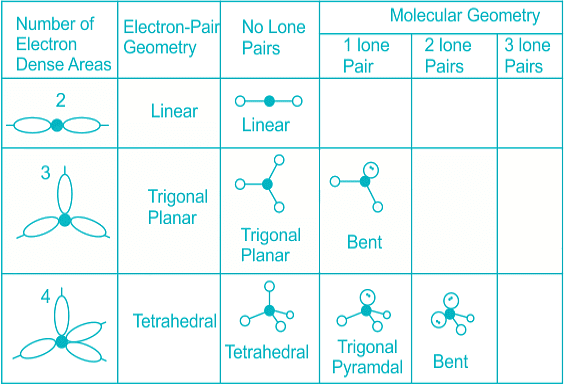
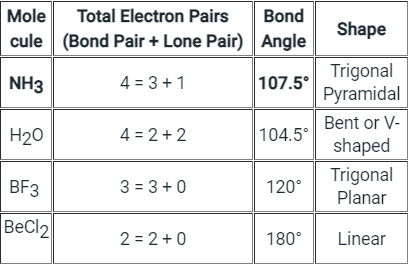
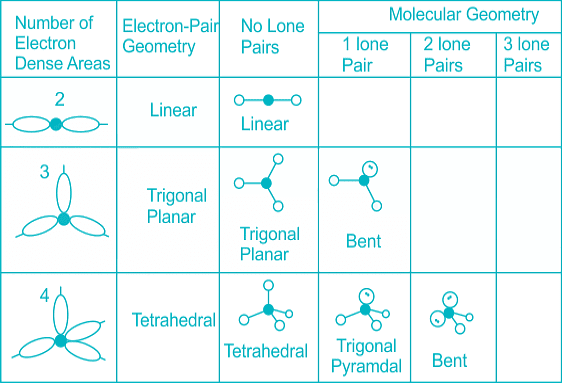
The below-mentioned table tells about the shape and bond angle of the compounds mentioned:
Hence we can conclude that Less than 109° but over 90° is the bond angle expected for H-N-H is NH3 molecule.
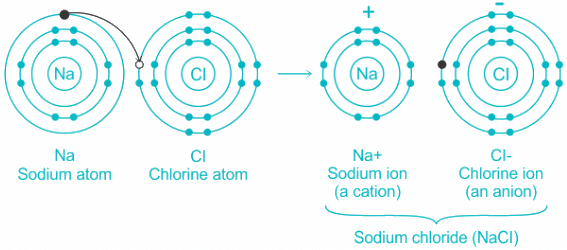
Q.3. Which type of bond is present in NaCl molecule
(a) Metallic
(b) Covalent
(c) Ionic
(d) None of the above
Correct Answer is Option (c)
Ionic Bond:
- Low positive charge and large size of cation and a small charge on anion and small size of anion favor the formation of ionic compounds.
- Ionic bond formation occurs via electrostatic attraction between two ions.
- One ion is positively charged and the other is negatively charged like in NaCl.
Hence, we can conclude that Ionic bond is present in NaCl molecule.
Covalent bonds:
- High positive charge and small size of cation and a high charge on anion and large size of anion favour the formation of covalent compounds.
- Covalent bond formation occurs when there is electron sharing occurs between two atoms.
- The electron pair is shared equally between the atoms forming the bond-like in Cl2.
Co-ordinate bond:
- It is formed by the complete donation of electrons from one atom to the other.
- The atom which donates the electron pair is called the donor atom.
- The atom accepting the electrons is called the acceptor.
Q.4. Which of the following pair has same geometry?
(a) CH4 and SF4
(b) NH4+ and XeF4
(c) CH4 and NH4+
(d) SF4 and XeF4+
Correct Answer is Option (c)
Molecular Geometry
- It is the 3D structure of atoms in a molecule and it determines the shape of a molecule around the central atom.
- It helps understand the entire atom and its arrangement.
- It is determined on the basis of only bonding electron pairs not the number of electron pairs.
- Valence shell electron pair repulsion theory provides a method for predicting the shape of molecules, based on the electron-pair electrostatic repulsion. According to VSEPR model, molecules adopt geometries in which their valence electron pair position themselves as far from each other as possible. This VSEPR model considers double bond and triple bond to have slightly greater repulsive effects than single bonds because of the repulsive effect of pi-electrons. However lone pairs create the maximum repulsive effect.
Explanation
- Geometry of CH4
The central atom C has 4 electron groups. All of them are bond pairs
There are 4 bond pairs and no lone pair of electrons.
So, according to VSEPR CH4 has a tetrahedral geometry.- Geometry of SF4
The central S atom has 5 electron groups, among them, 4 are bonding pairs and one lone pair.
So, According to VSEPR theory, SF4 has a see-saw geometry.- Geometry of NH4+
The central atom N has 4 electron pairs, These 4 electron pairs are used for bond formation.
So, a total of 4 bonding pairs and no lone pairs are present.
According to VSEPR theory, NH4+ has a tetrahedral geometry.- Geometry of XeF4
The central atom has 6 electron pairs among 4 of them are bond pairs 2 are lone pairs.
So, according to VSEPR theory XeF4 has square planar structure.
Among the given pair the same geometry is CH4 and NH4+ both the molecules has tetrahedral structure.
Q.5. The shapes of the compounds ClF3, XeOF2, N3− and XeO3F2 respectively, are:
(a) T-shape, T-shape, linear and trigonal bipyramidal
(b) trigonal planar, T-shape, V-shape and square pyramidal
(c) T-shape, trigonal planar, linear and square pyramidal
(d) trigonal planar, trigonal planar, V-shape and trigonal bipyramidal
Correct Answer is Option (a)
Concept
The geometry of a molecule-
- The geometry of a molecule depends on the arrangement of bonds about its centre in space.
- The arrangement further depends on the type of hybridization the centre atom is undergoing.
- The orientation of the hybrid orbitals is different in different cases.
- As bonds are formed via overlap of these orbitals, the bonds have directional nature.
- Therefore, hybridization is directly linked to the geometry of the molecule.
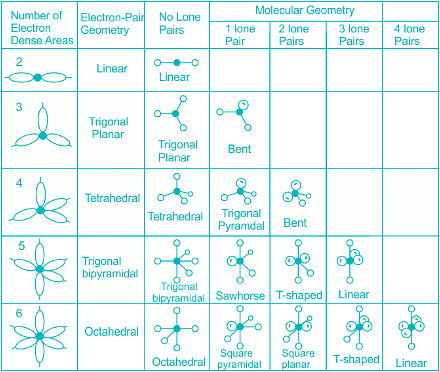
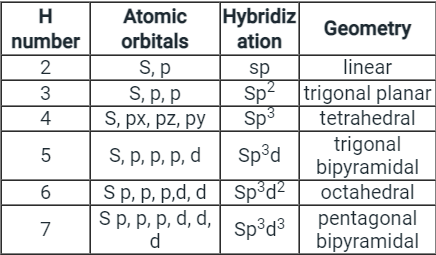
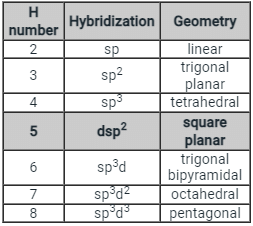
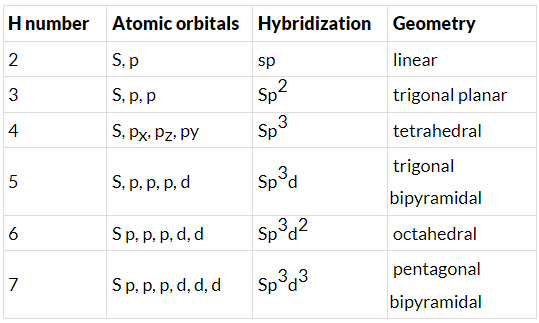
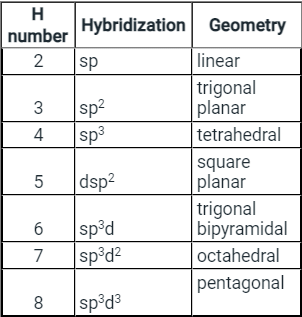
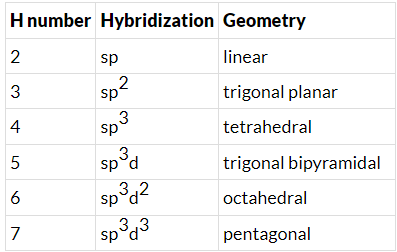
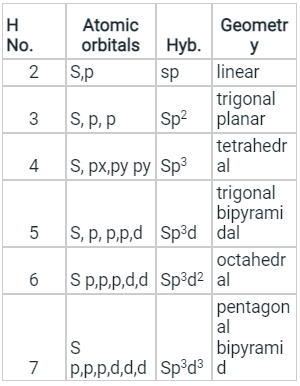
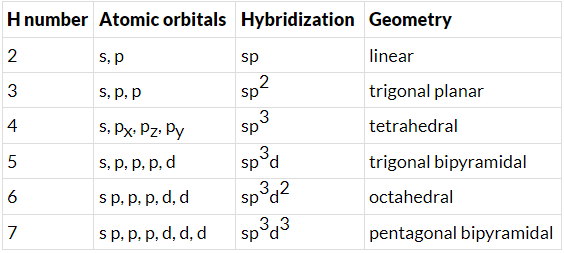
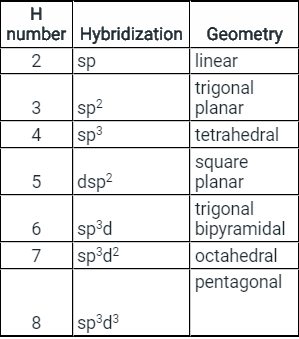
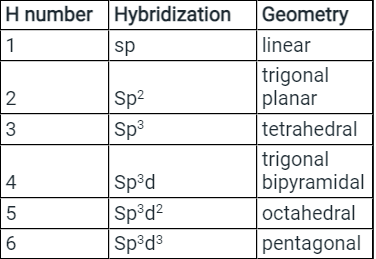
- Few types of hybridization, their modes of mixing, and geometry of molecules are-
Bents Rule:
- According to Bent's rule, the lone pair of electrons tend to occupy the position where there is more s- character and less p character.
- The electronegative substituents occupy positions where there is more p character.
- When multiple bonds are present, they occupy a position where there is more s-character.
Explanation:
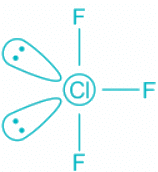
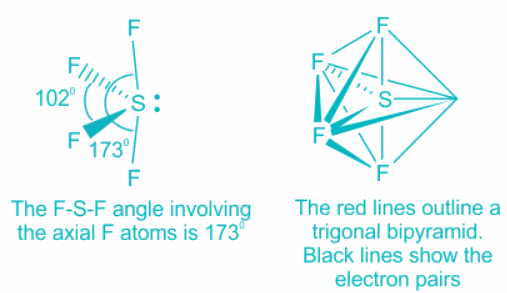
ClF3:
- The central atom chlorine contributes seven electrons towards hybridization and three F atoms contribute one electron each.
- This makes a total of 5 electron pairs and the corresponding hybridization trigonal bipyramidal.
- Out of the 5 electron pairs, two are lone pair and three are bond pairs.
- According to Bent's Rule, the lone pair will occupy the equatorial position as it has more s character and F will occupy the axial positions. Thus, the molecule will look like this:
- The shape of the molecule is thus T-shape which is also known as a see-saw.
XeOF2
- Contribution of electrons of Xe toward the bonding = 8
- Contribution from Fluorine = 2 , Oxygen = 2
- The total number of electron pairs is 6 out of which one is a pi bond, which corresponds to sp3d hybridization.
- Out of 5 are bond pairs, two are lone pairs. The lone pair and the double bond occupies the equatorial position following Bent's Rule.
- The shape of the molecule is T-Shape and the geometry trigonal pyramidal.
- Azide ion N3− is linear in shape.
XeO3F2:
- Contribution of electrons of Xe toward the bonding = 8
- Contribution from Fluorine = 2 , Oxygen = 2 × 3 = 6
- The total number of electron pairs is 8 out of which three are pi bonds.
- As pi bonds do not contribute towards hybridization, the number of electron pairs is 5, which corresponds to sp3d hybridization.
- All the double bond occupies the equatorial position following Bent's Rule. The geometry, as well as the shape, is trigonal bipyramidal.
Hence, the shapes of the compounds ClF3, XeOF2, N3− and XeO3F2 respectively, are T-shape, T-shape, linear and trigonal bipyramidal.
Q.6. Which type of bond is present in NaCl molecule
(a) Metallic
(b) Covalent
(c) Ionic
(d) None of the above
Correct Answer is Option (c)
Ionic Bond:
- Low positive charge and large size of cation and a small charge on anion and small size of anion favor the formation of ionic compounds.
- Ionic bond formation occurs via electrostatic attraction between two ions.
- One ion is positively charged and the other is negatively charged like in NaCl.
Hence, we can conclude that Ionic bond is present in NaCl molecule.
Covalent bonds:
- High positive charge and small size of cation and a high charge on anion and large size of anion favour the formation of covalent compounds.
- Covalent bond formation occurs when there is electron sharing occurs between two atoms.
- The electron pair is shared equally between the atoms forming the bond-like in Cl2.
Co-ordinate bond
- It is formed by the complete donation of electrons from one atom to the other.
- The atom which donates the electron pair is called the donor atom.
- The atom accepting the electrons is called the acceptor.
Q.7. The angle between two covalent bonds is maximum in:
(a) H2O
(b) CO2
(c) NH3
(d) CH4
Correct Answer is Option (b)
The geometry of a molecule:
- The geometry of a molecule depends on the arrangement of bonds about its center in space.
- The arrangement further depends on the type of hybridization the center atom is undergoing.
- The orientation of the hybrid orbitals is different in different cases.
- As bonds are formed via overlap of these orbitals, the bonds have directional nature.
- Therefore, hybridization is directly linked to the geometry of the molecule.
Hybridization and bond angles:
- According to VSEPR theory, the electron groups arrange themselves around each other so as to minimize repulsion.
- The electron group includes the bond pairs as well as lone pairs of electrons.
- If repulsion is more, the energy of the system is raised and the molecule becomes unstable.
- So, the arrangement in which there is minimum repulsion and maximum attraction is the most stable structure.
- The arrangement in space gives some angles between the central atom and the bonded atoms which are known as the bond angles.
- There exist anomalies in predicted bond angles when there is the presence of lone pair electrons.
Explanation:
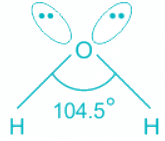
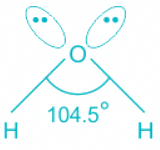
H2O:
- In water, the center atom oxygen is sp3 hybridized.
- Out of the three bond pairs, two are bond pairs and the other two are lone pairs.
- Due to the presence of lone electrons, there is repulsion between bond pairs and lone pairs.
- The repulsion between l.p is > b.p and thus pushes the two bonds to come closer decreasing the bond angle from ideal 109.8°.
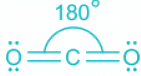
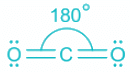
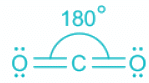
CO2:
- The bond between C and O atoms includes two pi and one sigma.
- A total of two sigma bonds means sp hybridization and linear geometry.
- In linear geometry, the bond angle is the highest at 180°.
- As the number of ligands about the central atom increases, the bond angle also decreases.
- But as linear geometry has the least steric, the bond angle is highest.
NH3:
- In ammonia, the center atom oxygen is sp3 hybridized.
- Out of the three bond pairs, three are bond pairs and the other is lone pair.
- Due to the presence of lone electrons, there is repulsion between bond pairs and lone pairs.
- The repulsion between l.p > b.p and thus pushes the two bonds to come closer decreasing the bond angle from ideal 109.8°
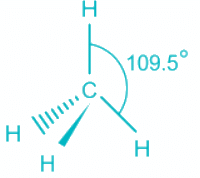
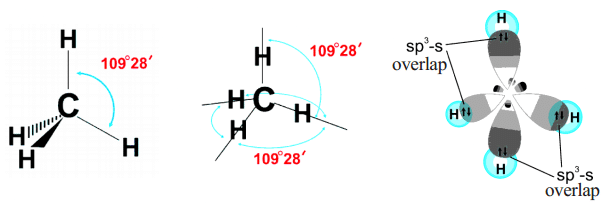
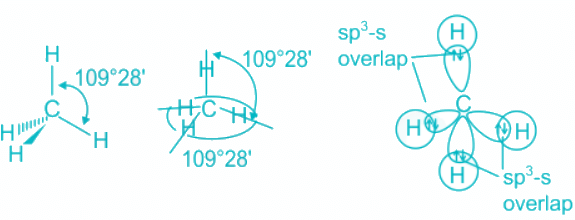
CH4
- CH4 molecule has sp3 hybridization.
- Each of these 4 sp3 hybrid orbitals overlaps with one 1s atomic orbital of hydrogen.
- The arrangement of the atoms gives tetrahedral geometry.
- There is no lone pair and the bond angle is ideal 109.5 °.
Thus, angle between two covalent bonds is maximum in CO2.
Important Points
- Distortion in shape occours when there is presence of lone pair of electrons as the table shows:
Q.8. The mode of hybridization of carbon in CO is
(a) sp
(b) sp2
(c) sp3
(d) None of these
Correct Answer is Option (a)
Concept
The geometry of a molecule:
- The geometry of a molecule depends on the arrangement of bonds about its centre in space.
- The arrangement further depends on the type of hybridization the centre atom is undergoing.
- The orientation of the hybrid orbitals is different in different cases.
- As bonds are formed via overlap of these orbitals, the bonds have directional nature.
- Therefore, hybridization is directly linked to the geometry of the molecule.
Hybridization and bond angles:
- According to VSEPR theory, the electron groups arrange themselves around each other so as to minimize repulsion.
- The electron group includes the bond pairs as well as lone pairs of electrons.
- If repulsion is more, the energy of the system is raised and the molecule becomes unstable.
- So, the arrangement in which there is minimum repulsion and maximum attraction is the most stable structure.
- The arrangement in space gives some angles between the central atom and the bonded atoms which are known as the bond angles.
Few types of hybridization, their modes of mixing, and geometry of molecules are-
Explanation:
- In CO, the structure is: C ≡ O :
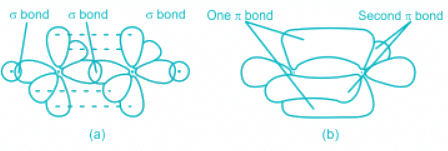
- There exist three bonds between carbon and oxygen out of which one is sigma and two are pi.
- So, two pi bond corresponds to 'sp' hybridization.
- The sigma bond between the two atoms is made by the head-on overlap of 2p - 2p orbitals of Carbon and Oxygen.
- The pi bonds are formed by the lateral overlap between p orbitals of carbon and oxygen.
Hence, the mode of hybridization of carbon in CO is 'sp'.Additional Information
sp Hybridisation of carbon:
- The bond between two C atoms includes two pi and one sigma.
- A pi bond is formed by pure p-p overlap.
- One sigma bond exists between each carbon and hydrogen.
- A total of two sigma bonds means sp hybridization and linear geometry.
sp2 Hybridisation of carbon:
- The bond between two C atoms includes one pi and one sigma.
- A pi bond is formed by pure p-p overlap.
- This type of hybridization is seen in alkenes.
- The geometry is trigonal planar.
sp3 Hybridisation of carbon:
- Only sigma bonds are formed by the carbon atoms.
- The geometry is tetrahedral.
Q.9. The nitrogen molecule has:
(a) 1 σ and 1 π bond
(b) 2 σ and 2 π bond
(c) 2 σ and 1 π bond
(d) 1 σ and 2 π bond
Correct Answer is Option (d)
Sigma bonds:
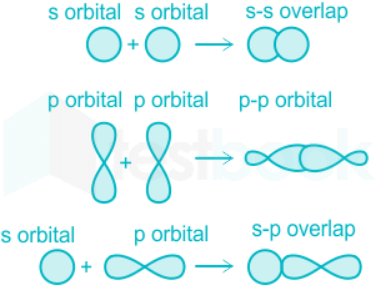
- These are the type of covalent bonds formed by the head-on overlap of atomic orbitals.
- Sigma bonds can be formed by the overlap between-
- s and s orbital
- s and p orbital
- p and p orbital.
- The extent of overlap in the case of sigma bonds is to a high extent.
Pi bonds:
- These bonds are formed by a lateral overlap between atomic orbitals.
- The lateral overlap between two s orbitals is not possible due to its symmetrical shape, however is possible in the case of p orbitals.
Explanation:
- Nitrogen has three bond between two N atoms.
- When there are more than one bond in between two molecules, one is sigma and the other are pi bonds.
- There is triple bond in N2 molecule out of which one is sigma and two pi bonds.
- Hence, the nitrogen molecule has 1 σ and 2 π bond.
Q.10. dsp2 hybridization is in
(a) PCl5
(b) [Ni(CN)4]2-
(c) Ni(CO)4
(d) None of these
Correct Answer is Option (b)
Concept
Crystal field theory:
- A metal ion in a complex is surrounded by coordinating ligands anions or negative ends.
- An electric field is produced at the metal ion by the surrounding ligands.
- This electrostatic approach towards the interaction between metals and ligands is known as crystal field theory.
- CFT theory considers the ligands as point charges.
- There is no overlap between the ligand orbitals and metal ion orbitals.
- Ligands donate lone pair of electrons to the metal atoms and form coordinate complexes.
The geometry of the complex formed:
- Placement of the donor atoms at different stereochemical positions will perturb the metal ions to a different extent.
- This will result in the formation of different stereochemistries or structures.
- The stereochemistry also depends on the coordination number and the type of orbitals involved.
Explanation:
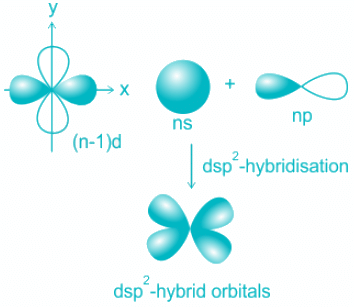
- The formation of square planar complexes involves one s, two p, and dx2-y2 orbitals.
- Four pure orbitals mix and results in the formation of four dsp2 orbitals.
- The four orbitals are distributed along with four corners of a square plane as shown above.
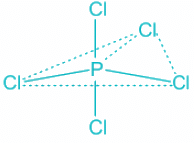
PCl5
- In the PCl5 molecule, phosphorus is the central atom.
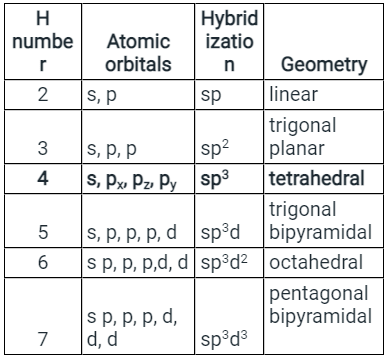
- H = ½ (V + M - C + A ) V = number of valence electrons on the phosphorus atom = 5 M = No. Of monovalent atoms = 5 C = cationic charge =,0 A = anionic charge = 0
- Putting this in the formula, we get H = 5, which corresponds to sp3d hybridization. The structure of the PCl5 molecule is:
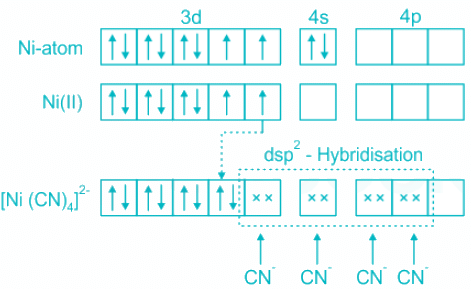
[Ni(CN)4]2- :
- Nickel (Ni) in the complex [NiCl4]2- is in a +2 oxidation state. Hence the electronic configuration is [Ar]3d8.
- Out of 8 3d electrons, 6 are paired and two are unpaired.
- As Cyanide is a strong field ligand, it pairs up the electrons in Ni2+ and makes available one inner 3d orbital.
- The orbitals participating are 3d, 4s, and 4p resulting in hybridization dsp2.
- The shape is square planar.
Ni(CO)4:
- Nickel (Ni) in the complex Ni(CO)4 is in a 0oxidation state. Hence the electronic configuration is [Ar]3d10.
- There is no vacant d orbital as 3d orbitals are fulfilled.
- Thus, dsp2 hybridisation is not possible for Ni(CO)4. It is tetrahedral.
Hence, dsp2 hybridization is in [Ni(CN)4]2-.
Q.11. The atomic number of magnesium is 12 and mass number is 24. Which one of the following is the correct representation of the ion formed from it and its valency?
(a) Mg2+ ; valency = 2
(b) Mg2+ ; valency = 6
(c) Mg3+ ; valency = 5
(d) Mg3+ ; valency = 3
Correct Answer is Option (a)
Concept
- Valance Electrons: Valence electron is simply the total number of electrons present in the outermost shell of an atom.
- Valency: Valency is the number of electrons an atom loses, gains or shares with other atoms in order to get a stable configuration.
For example-
Atomic Configuration of Carbon (C) is (6) = 2, 4
- Valency of Carbon will be 4 as it needs 4 more electrons to get a stable configuration.
- Number of valence electrons in carbon is also four as it's outermost shell contains 4 electrons.
Atomic Configuration of Nitrogen N ( 7 ) = 2, 5
Valency of nitrogen is 3 as it needs 3 more electrons to complete its octet but number of valence electrons in nitrogen is 5.Explanation
- Atomic Number of Magnesium (Mg) is - 12
- Atomic Configuration of Mg = 2, 8, 2
- Its valance electrons is less than 4.
- So, its Valancy is 2 i.e electrons in the last cell.
- Therefore the stable configuration of Mg will be when it loses 2 electrons i.e. Mg 2+
Q.12. Which is not a linear molecule?
(a) CO2
(b) C2H2
(c) HCN
(d) CH4
Correct Answer is Option (d)
Concept
Hybridization:
- The atomic orbitals s, p, d, f of the central atom having compatible energies mix up.
- This leads to the formation of new hybrid orbitals which have now different energies, shapes, and orientations than the component atomic orbitals.
- The number of hybrid orbitals is equal to atomic orbitals mixing.
- After the formation of hybrid orbitals, they overlap with atomic orbitals of suitable side atoms and leads to the formation of covalent chemical bonds.
The geometry of a molecule-
- The geometry of a molecule depends on the arrangement of bonds about its center in space.
- The arrangement further depends on the type of hybridization the center atom is undergoing.
- The orientation of the hybrid orbitals is different in different cases.
- As bonds are formed via overlap of these orbitals, the bonds have directional nature.
- Therefore, hybridization is directly linked to the geometry of the molecule.
Few types of hybridization, their modes of mixing, and geometry of molecules are-
Explanation
- In CO2, C2H2, HCN the central carbon undergoes sp hybridization and forms two pi and two sigma bonds.
- Two sigma bonds are formed by two sp hybrid orbitals.
- All of them have a linear geometry.
CH4
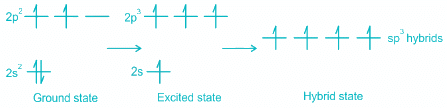
- While in CH4, the central atom undergoes hybridization involving one 2s and three 2p atomic orbitals.
- In the excited state, each orbital is singly occupied.
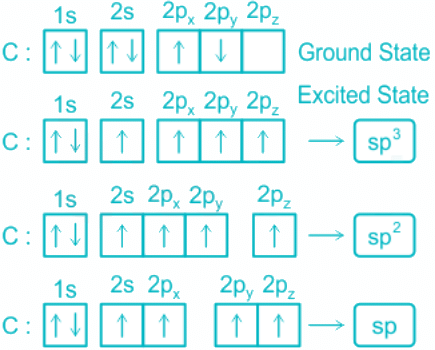
- These four atomic orbitals mix and forms four sp3 hybrid orbitals, shown below:
- Each of these 4 sp3 hybrid orbitals overlaps with one 1s atomic orbital of hydrogen.
- The arrangement of the atoms gives tetrahedral geometry.
Hence, methane is not a linear molecule.
Additional Information
- The structures of CO2, C2H2 and HCN are shown below:
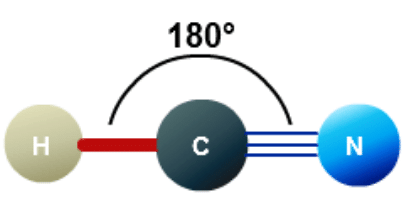
Q.13. What is the bond angle expected for H-N-H is NH3 molecule?
(a) 120°
(b) Over 109° but the less than 120°
(c) 109.5°
(d) Less than 109° but over 90°
Correct Answer is Option (d)
Concept
- The Valence Shell Electron Pair Repulsion (VSEPR) Theory provides the procedure to determine the shape of the covalent molecule.
- The electron pair that is involved in the bonding is termed as the bond pair, while the one not involved in bonding is termed as the lone pair of electron.
- The ammonia molecule has a trigonal pyramidal shape with the three hydrogen atoms and an unshared pair of electrons attached to the nitrogen atom.
Key Points
NH3 Bond angles:
- There are three single bonds and one lone pair of electrons in NH3 molecule.
- It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure.
- The shape is distorted because of the lone pairs of electrons.
- This pair exerts repulsive forces on the bonding pairs of electrons.
- Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it decreases to 107 degrees due to the lone pair on the nitrogen atom.
The below-mentioned table tells about the shape and bond angle of the compounds mentioned:
Hence we can conclude that Less than 109° but over 90° is the bond angle expected for H-N-H is NH3 molecule.
Q.14. Pick out the isoelectronic structure from the Following:
I. CH3+, II. H3O+, III. NH3, IV. CH3-
(a) I and II
(b) III and IV
(c) I and III
(d) II, III, IV
Correct Answer is Option (d)
Concept
Isoelectronic species :
- Those species having the same number of electrons but differ in nuclear charge are called isoelectronic species.
- Example: Mg2+, Na+, F–, and O2– have 10 electrons each so they are isoelectronic.
- But, their radius is different due to differences in nuclear charges.
- Ionic radius decreases along a period for Isoelectronic ions.
Isostructural species:
- The species which have different atoms or elements in them but have the same hybridization and structure are called isostructural species.
- Examples are NF3 and NH3, they have both sp3 hybridization and pyramidal shape.
Explanation
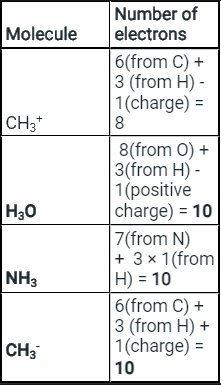
- The species and their structures and number of electrons are given below:
- From the table above we see that CH3-, NH3, H3O+ all have 10 electrons in them.
- Hence, they are isoelectronic species.
Thus the correct option is II, III, IV.
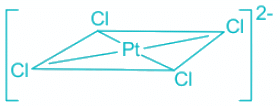
Q.15. What is the shape of [PtCl4]2- ?
(a) Tetrahedron
(b) See-Saw
(c) Square planar
(d) Square pyramid
Correct Answer is Option (c)
Concept
Crystal field theory:
- A metal ion in a complex is surrounded by coordinating ligands anions or negative ends.
- An electric field is produced at the metal ion by the surrounding ligands.
- This electrostatic approach towards the interaction between metals and ligands is known as crystal field theory.
- CFT theory considers the ligands as point charges.
- There is no overlap between the ligand orbitals and metal ion orbitals.
- Ligands donate lone pair of electrons to the metal atoms and form coordinate complexes.
The geometry of the complex formed.
- Placement of the donor atoms at different stereochemical positions will perturb the metal ions to a different extent.
- This will result in the formation of different stereochemistries or structures.
- The stereochemistry also depends on the coordination number and the type of orbitals involved.
Explanation
[PtCl4]2- :
- Platinum (Pt) in the complex [PtCl4]2- is in a +2 oxidation state. Hence the electronic configuration is [Xe]4f145d8.
- Out of 8 5d electrons, 6 are paired and two are unpaired in the ground state.
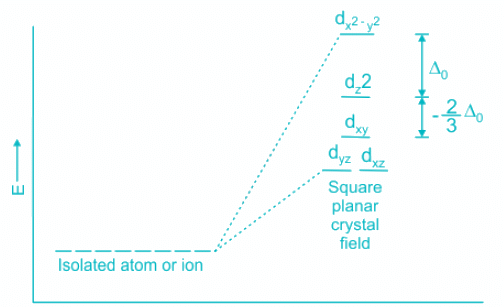
- The CFSE of a square planar complex is:
- In presence of CFSE, the degeneracy of the dx2-y2 and dz2 breaks further.
- Thus, all the electrons gets paired leaving one of the d orbitals vacant.
- Hence, inner 5d, 6s and 6p orbitals are involved in hybridisation.
- The resulting hybridisation is dsp2 and the geometry is square planar.
Hence, the shape of [PtCl4]2- is square planar.
Q.16. The VSEPR Model is used for predicting the _______ of the molecules.
(a) Geometrical shapes
(b) Bond length
(c) Bond order
(d) Number of resonating structure
Correct Answer is Option (a)
Key Points
- The VSEPR model used for predicting the geometrical shapes of molecules is based on the assumption that electron pairs repel each other and, therefore, tend to remain as apart as possible.
- According to this model, molecular geometry is determined by repulsions between lone pairs and lone pairs, lone pairs and bond pairs and bond pairs and bond pairs.
- The order of these repulsions being: lone pairs - lone pairs > lone pairs - bond pairs > bond pairs - bond pairs.
- Sidgwick and Powell in the year 1940, proposed a simple theory based on the repulsive interactions of the electron pairs in the valence shell of the atoms.
- The VSEPR theory can predict the geometry of a large number of molecules, especially the compounds of p-block elements accurately.
- It is also quite successful in determining the geometry quite-accurately even when the energy difference between possible structures is very small.
Additional Information
- Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule.
- Bond lengths are measured by spectroscopic, X-ray diffraction, and electron diffraction techniques.
- Bond order is the number of bonds between the two atoms in a molecule.
- In the Lewis description of the covalent bond, the bond Order is given by the number of bonds between the two atoms in a molecule.
- With an increase in bond order, bond enthalpy increases and 5 decreases.
- The concept of resonance says that whenever a single Lewis structure cannot describe a molecule accurately, a number of structures with similar energy, positions of nuclei, bonding, and non-bonding pairs of electrons are taken as the canonical structures of the hybrid which describes the molecule accurately.
Q.17. What is the hybridisation of the oxygen atom in water?
(a) sp
(b) sp2
(c) sp3
(d) dsp2
Correct Answer is Option (c)
Concept:
Hybridization:
- The atomic orbitals s, p, d, f of the central atom having compatible energies mix up.
- This leads to the formation of new hybrid orbitals which have now different energies, shapes, and orientations than the component atomic orbitals.
- The number of hybrid orbitals is equal to atomic orbitals mixing.
- After the formation of hybrid orbitals, they overlap with atomic orbitals of suitable side atoms and leads to the formation of covalent chemical bonds.
- The formula for calculating hybridization - H = ½ ( V + M -C + A)
- Where V = number of valence electrons on the central atom. M = No. Of monovalent atoms C = cationic charge A = anionic charge.
Few types of hybridization, their modes of mixing, and geometry of molecules are-
Explanation
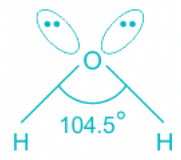
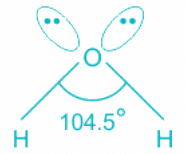
H2O:
- Oxygen is the central molecule.
- Oxygen donates six electrons towards hybridization and one electron is donated by each hydrogen atom.
- So total number of electrons = 2 + 6 = 8 and number of pairs = 4. Hence, H = 4 and the hybridisation is sp3.
- Out of four pairs, two are bond pairs and two are lone pairs.
- The expected geometry is tetrahedral but the actual shape is bent.
Hence, the hybridization of the oxygen atom in water is sp3.
Additional Information
- Due to the presence of lone electrons, there is repulsion between bond pairs and lone pairs.
- The repulsion between l.p is > b.p and thus pushes the two bonds to come closer decreasing the bond angle from ideal 109.8°.
Q.18. Which of the following molecules represent the type of hybridization sp3, sp2, sp2, sp from left to right atoms?
(a) CH ≡ C - C ≡ CH
(b) CH2 = CH - C ≡ CH
(c) CH3 - CH = CH - CH3
(d) CH3 - CH = CH - CN
Correct Answer is Option (d)
Concept
Hybridization:
- The atomic orbitals s, p, d, f of the central atom having compatible energies mix up.
- This leads to the formation of new hybrid orbitals which have now different energies, shapes, and orientations than the component atomic orbitals.
- The number of hybrid orbitals is equal to atomic orbitals mixing.
- After the formation of hybrid orbitals, they overlap with atomic orbitals of suitable side atoms and leads to the formation of covalent chemical bonds.
- When there exists a single bond between two atoms, there is only a sigma bond which is formed by hybrid orbitals.
- When there exist multiple bonds between atoms, there is only one sigma bond and others are pi bond.
- sp Hybridisation:
- When one ‘s’ orbital and one ‘p’ orbitals of an atom mix to form two new equivalent orbitals.
- sp2 Hybridisation:
- When one ‘s’ orbital and two ‘p’ orbitals of an atom mix to form 3 equivalent orbitals.
- sp3 Hybridisation:
- When one ‘s’ orbital and three ‘p’ orbitals of an atom mix together to form four new equivalent orbital.
Explanation
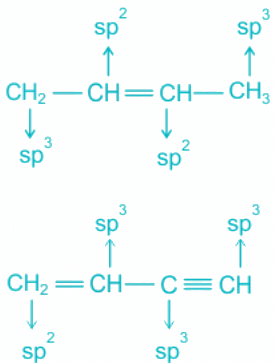
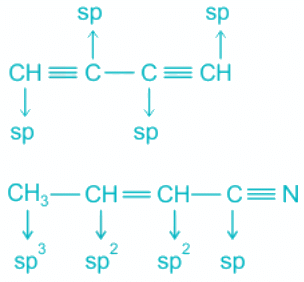
sp Hybridisation of carbon:
- The bond between two C atoms includes two pi and one sigma.
- A pi bond is formed by pure p-p overlap.
- One sigma bond exists between each carbon and hydrogen.
- A total of two sigma bonds means sp hybridization and linear geometry.
sp2 Hybridisation of carbon:
- The bond between two C atoms includes one pi and one sigma.
- A pi bond is formed by pure p-p overlap.
- This type of hybridization is seen in alkenes.
- The geometry is trigonal planar.
sp3 Hybridisation of carbon:
- Only sigma bonds are formed by the carbon atoms.
- The geometry is tetrahedral.
- The pi and sigma bonds in the molecules are shown as follows:
Hence, the molecule representing the type of hybridization sp3, sp2, sp2, sp from left to right atoms is CH3 - CH = CH - CN.
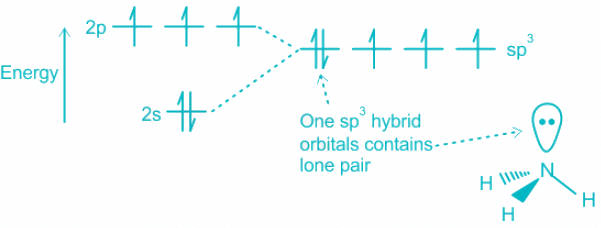
Q.19. Structure of ammonia is:
(a) Pyramidal
(b) Tetrahedral
(c) Trigonal
(d) Trigonal bipyramidal
Correct Answer is Option (b)
Concept
Hybridization:
- The atomic orbitals s, p, d, f of the central atom having compatible energies mix up.
- This leads to the formation of new hybrid orbitals which have now different energies, shapes, and orientations than the component atomic orbitals.
- The number of hybrid orbitals is equal to atomic orbitals mixing.
- After the formation of hybrid orbitals, they overlap with atomic orbitals of suitable side atoms and leads to the formation of covalent chemical bonds.
- The formula for calculating hybridization - H = ½ (V + M -C + A)
- Where V = number of valence electrons on the central atom. M = No. Of monovalent atoms C = cationic charge A = anionic charge.
Few types of hybridization, their modes of mixing, and geometry of molecules are-
Explanation
- Contribution of electrons toward bonding by Ammonia = 5, H = 3
- A total number of electron pairs = 4; which corresponds to sp3 hybridization.
- The hybridization of the orbitals in ammonia takes place as follows:
(i) One of the electrons from 2s is promoted to 2p orbital.
(ii) The four atomic orbital 2s and three 2p create four sp3 hybrid orbitals.
(iii) One lone pair resides on the sp3 and the others form bonds overlapping with 1s orbital of Hydrogen.Mistake Points
- Note that the presence of lone pair creates distortion in geometry.
- The actual shape of the molecule is pyramidal and geometry is tetrahedral.
Q.20. Which of the following is a linear molecule
(a) H2O
(b) SO2
(c) CO2
(d) H2S
Correct Answer is Option (c)
Concept:
Hybridization
- The atomic orbitals s, p, d, f of the central atom having compatible energies mix up.
- This leads to the formation of new hybrid orbitals which have now different energies, shapes, and orientations than the component atomic orbitals.
- The number of hybrid orbitals is equal to atomic orbitals mixing.
- After the formation of hybrid orbitals, they overlap with atomic orbitals of suitable side atoms and leads to the formation of covalent chemical bonds.
- When there exists a single bond between two atoms, there is only a sigma bond that is formed by hybrid orbitals.
- When there exist multiple bonds between atoms, there is only one sigma bond and others are pi bond.
The geometry of a molecule-
- The geometry of a molecule depends on the arrangement of bonds about its center in space.
- The arrangement further depends on the type of hybridization the center atom is undergoing.
- The orientation of the hybrid orbitals is different in different cases.
- As bonds are formed via overlap of these orbitals, the bonds have directional nature.
- Therefore, hybridization is directly linked to the geometry of the molecule.
- Few types of hybridization, their modes of mixing, and geometry of molecules are-
Explanation
- SO2 molecule, sulfur is the central atom.
H = ½ (V + M - C + A)
- Putting this in the formula, we get H = 3, which corresponds to sp2 hybridization. The structure of the SO2 molecule is pyramidal.
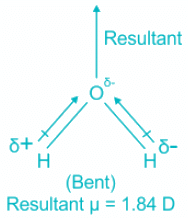
H2O
- The central element in water is oxygen. The molecule has sp3 hybridization where two are bond pairs and two are lone pairs.
- The shape of the molecule is bent.
CO2
- The central element is carbon. It is a neutral molecule.
- The electronic contribution from carbon is 4 and from two oxygen is 4. The total electronic pair is thus 4 out of which two are sigma bonds are two are pi bonds.
- As pi bonds do not contribute toward hybridization, the total electronic pairs are two.
- Two-electron pairs mean sp hybridization and the shape is linear.
H2S
- The central element in water is sulfur. The molecule has sp3 hybridization where two are bond pairs and two are lone pairs.
- The shape of the molecule is bent.
Hence, the linear molecule is CO2.
- Pi bonds do not contribute towards hybridization, only sigma bond does.
- When lone pairs are present in a molecule, the geometry gets distorted from ideal geometry.
Q.21. What is the hybridisation of carbon atom in methane?
(a) sp
(b) sp2
(c) sp3
(d) dsp2
Correct Answer is Option (c)
Concept
Hybridization:
- The atomic orbitals s, p, d, f of the central atom having compatible energies mix up.
- This leads to the formation of new hybrid orbitals which have now different energies, shapes, and orientations than the component atomic orbitals.
- The number of hybrid orbitals is equal to atomic orbitals mixing.
- After the formation of hybrid orbitals, they overlap with atomic orbitals of suitable side atoms and leads to the formation of covalent chemical bonds.
The geometry of a molecule-
- The geometry of a molecule depends on the arrangement of bonds about its centre in space.
- The arrangement further depends on the type of hybridization the center atom is undergoing.
- The orientation of the hybrid orbitals is different in different cases.
- As bonds are formed via overlap of these orbitals, the bonds have directional nature.
- Therefore, hybridization is directly linked to the geometry of the molecule.
Few types of hybridization, their modes of mixing, and geometry of molecules are-
Explanation
sp Hybridisation of carbon:
- The bond between two C atoms includes two pi and one sigma.
- A pi bond is formed by pure p-p overlap.
- One sigma bond exists between each carbon and hydrogen.
- A total of two sigma bonds means sp hybridization and linear geometry.
sp2 Hybridisation of carbon:
- The bond between two C atoms includes one pi and one sigma.
- A pi bond is formed by pure p-p overlap.
- This type of hybridization is seen in alkenes.
- The geometry is trigonal planar.
sp3 Hybridisation of carbon:
- Only sigma bonds are formed by the carbon atoms.
- The geometry is tetrahedral.
CH4
- While in CH4, the central atom undergoes hybridization involving one 2s and three 2p atomic orbitals.
- In the excited state, each orbital is singly occupied.
- These four atomic orbitals mix and forms four sp3 hybrid orbitals, shown below:
- Each of these 4 sp3 hybrid orbitals overlaps with one 1s atomic orbital of hydrogen.
- The arrangement of the atoms gives tetrahedral geometry.
Hence, the hybridisation of carbon atom in methane is sp3.
Q.22. Which of the following has sp3d hybridization?
(a) NH+4
(b) SF4
(c) XeF4
(d) XeOF4
Correct Answer is Option (b)
Concept:
Hybridization:
- The atomic orbitals s, p, d, and f of the central atom having compatible energies mix up.
- This leads to the formation of new hybrid orbitals which have now different energies, shapes, and orientations than the component atomic orbitals.
- The number of hybrid orbitals is equal to atomic orbitals mixing.
- After the formation of hybrid orbitals, they overlap with atomic orbitals of suitable side atoms and lead to the formation of covalent chemical bonds.
- The formula for calculating hybridization - H = ½ ( V + M -C + A)
- Where V = number of valence electrons on the central atom. M = No. Of monovalent atoms C = cationic charge A = anionic charge.
Few types of hybridization, their modes of mixing, and the geometry of molecules are-
Explanation:
SF4
- Contribution of electrons of sulfur toward the bonding = 6
- Contribution from Fluorine = 4
- The total number of electron pairs is 5, which corresponds to sp3d hybridization.
- However, out of these 5 pairs, 4 are bond pairs and one is lone pair.
- The geometry is trigonal bipyramidal but the shape is a see-saw.
Hence, SF4 has sp3d hybridization.
Additional Information
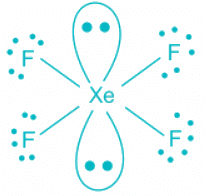
XeF4
- Contribution of electrons of Xe toward the bonding = 8
- Contribution from Fluorine = 4
- The total number of electron pairs is 6, which corresponds to sp3d2 hybridization.
- Out of 6 pairs, 4 are bond pairs and two are lone pairs.
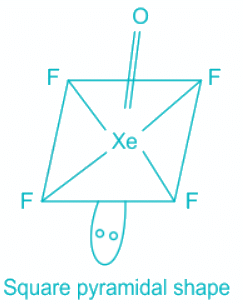
XeOF4
- Contribution of electrons of Xe toward the bonding = 8
- Contribution from Fluorine = 4 , Oxygen = 2
- The total number of electron pairs is 7 out of which one is a pi bond, which corresponds to sp3d2 hybridization.
- Out of 6 pairs, 5 are bond pairs, and one is a lone pair and the shape is square pyramidal.
NH4+
- Contribution of electrons toward bonding by Ammonia = 5 , H = 3
- A total number of electron pairs = 4; which corresponds to sp3 hybridization.
- There are three bond pairs and one lone pair in ammonia.
- The lone pair is donated to an H+ ion to form a co-ordinate bond. It occupies the fourth position of a tetrahedron,
Q.23. Which has a minimum bond angle?
(a) H2O
(b) H2S
(c) NH3
(d) CH4
Correct Answer is Option (b)
Content:
The geometry of a molecule:
- The geometry of a molecule depends on the arrangement of bonds about its center in space.
- The arrangement further depends on the type of hybridization the center atom is undergoing.
- The orientation of the hybrid orbitals is different in different cases.
- As bonds are formed via overlap of these orbitals, the bonds have directional nature.
- Therefore, hybridization is directly linked to the geometry of the molecule.
Hybridization and bond angles:
- According to VSEPR theory, the electron groups arrange themselves around each other so as to minimize repulsion.
- The electron group includes the bond pairs as well as lone pairs of electrons.
- If repulsion is more, the energy of the system is raised and the molecule becomes unstable.
- So, the arrangement in which there are minimum repulsion and maximum attraction is the most stable structure.
- The arrangement in space gives some angles between the central atom and the bonded atoms which are known as the bond angles.
- There exist anomalies in predicted bond angles when there is the presence of lone pair electrons.
Explanation:
H2O:
- In water, the center atom oxygen is sp3 hybridized.
- Out of the four bond pairs, two are bond pairs and the other two are lone pairs.
- Due to the presence of lone electrons, there is repulsion between bond pairs and lone pairs.
- The repulsion between l.p is > b.p and thus pushes the two bonds to come closer decreasing the bond angle from ideal 109.8°.
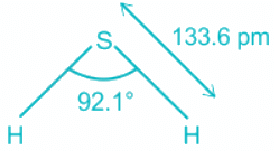
H2S:
- In water, the center atom oxygen is sp3 hybridized.
- Out of the three bond pairs, two are bond pairs and the other two are lone pairs.
- Due to the presence of lone electrons, there is repulsion between bond pairs and lone pairs.
- The repulsion between l.p is > b.p and thus pushes the two bonds to come closer decreasing the bond angle from ideal 109.8°.
- As sulfur is a larger molecule than oxygen, its electron density is more dispersed. It is more easily distorted by the repulsion from the lone pair of electrons and the bond pairs come closer to each other.
- This decreases the bond angle more than in water.
- The bond angle in H2S is 92.1°.
NH3:
- In ammonia, the center atom oxygen is sp3 hybridized.
- Out of the three bond pairs, three are bond pairs and the other is lone pair.
- Due to the presence of lone electrons, there is repulsion between bond pairs and lone pairs.
- The repulsion between l.p > b.p and thus pushes the two bonds to come closer decreasing the bond angle from ideal 109.8° to 107°.
CH4
- CH4 molecule has sp3 hybridization.
- Each of these 4 sp3 hybrid orbitals overlaps with one 1s atomic orbital of hydrogen.
- The arrangement of the atoms gives tetrahedral geometry.
- There is no lone pair and the bond angle is ideal 109.5 °.
Hence, the minimum bond angle is present in H2S.
Important Points
- Distortion in shape occurs when there is presence of lone pair of electrons as the table shows:
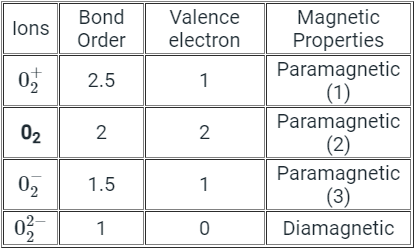
Q.24. Oxygen molecule exhibits
(a) Diamagnetism
(b) Paramagnetism
(c) Ferromagnetism
(d) Antiferromagnetism
Correct Answer is Option (b)
Concept
Paramagnetic materials
- Small, positive susceptibility to magnetic fields
- These materials are slightly attracted by a magnetic field
- Paramagnetic properties are due to the presence of some unpaired electrons, and from the realignment of the electron paths caused by the external magnetic field
- Paramagnetic materials include magnesium, molybdenum, lithium, and tantalum
Explanation
According to molecular orbital theory O2 molecule is more paramagnetic because it has two unpaired electrons in antibonding molecular orbital.The neutral oxygen is paramagnetic according to MO theory because it ends up with two unpaired electrons in two degenerate pi antibonding molecular orbitals.
The other two are paramagnetic because they have an odd number of electrons so it doesn’t matter what kind of bonding they are involved in, the electrons cannot be all paired up.these would also be diamagnetic as the double negative would have filled up the pi* orbitals and the double-positive version would have left both of them empty.
|
50 videos|92 docs|41 tests
|
FAQs on Solved Practice Questions on VSEPR Theory and Shapes of Molecules - Inorganic Chemistry
| 1. What is VSEPR theory and how does it explain the shapes of molecules? |  |
| 2. How do lone pairs of electrons affect the molecular shape according to VSEPR theory? |  |
| 3. Can VSEPR theory predict the bond angles in a molecule? |  |
| 4. What is the difference between molecular shape and electron domain geometry according to VSEPR theory? |  |
| 5. Can VSEPR theory predict the polarity of a molecule? |  |