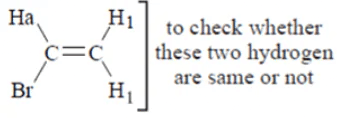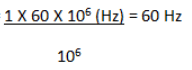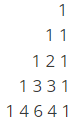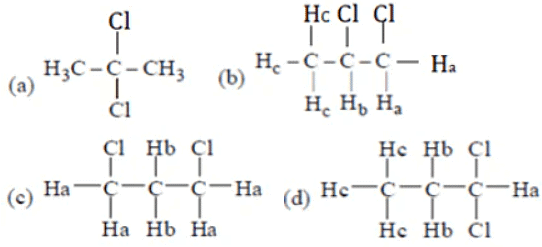Solved Practice Questions on NMR Spectroscopy | Organic Chemistry PDF Download
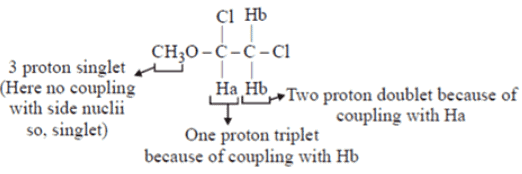
Q.1. The 1H NMR spectrum of CH3OCHCICH2Cl will exhibit _________.
(a) A three proton doublet. One proton singlet and a two proton doublet
(b) A three proton singlet. One proton singlet and a two proton doublet
(c) A three proton singlet. One proton triplet and a two proton doublet
(d) A three proton triplet. One proton triplet and a two proton triplet
Correct Answer is Option (c)
The 1H NMR spectrum of CH3OCHCICH2Cl will exhibit three proton singlet, one proton triplet and two proton doublet.
No. of peaks = n + 1
(n = no. of neighbouring chemically equivalent Hydrogen nuclii)
Q.2. Which of the following has three types of hydrogens in the following compounds?
(a) Br-CH = CH2
(b) CH3 – CH2 – CH3
(c) C6H5CH2
(d) CH3– CH2 – CH(CH3) – N02
Correct Answer is Option (a)
We will replace them by D and check the structure if they are different then they are different.
both are different
so, total 3 type of hydrogen
So, three different type of hydrogen is present in compound a.
Q.3. How many Hertz does 1 ppm correspond to for an PMR spectrometer operating at a radio frequency of 60 MHz and 100 MHz?
(a) 6 Hz, 10 Hz
(b) 60 Hz, 100 Hz
(c) 100 Hz, 60 Hz
(d) 10Hz, 100Hz
Correct Answer is Option (b)
Chemical shift (ppm) =
For 60 MHz; frequency (Hz) =
Similarly, for 100 MHz, Frequency (Hz) = 100 Hz.
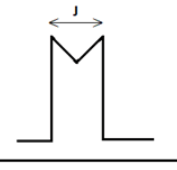
Q.4. The distance between the centers of the peaks of doublet is called as?
(a) Coupling constant
(b) Spin constant
(c) Spin-spin coupling
(d) Chemical shift
Correct Answer is Option (a)
When the proton under observation is split into several peaks by neighboring protons, the distance between these peaks is a constant called the coupling constant (J). Any two sets of protons that exhibit the same coupling constant are most likely splitting eachother signals and are said to be coupled.
Q.5. A proton Hb is coupled to four equivalent protons Ha. The multiplicity and the relative intensity of lines in the signal Hb is?
(a) Doublet, I : 4
(b) Triplet, I : 4 : 6
(c) Quintet, 1 : 4 : 6 : 4 : 1
(d) Quartet, 1 : 4 : 6 : 4
Correct Answer is Option (c)
Each signal in a proton NMR spectrum may or may not be split into one or more peaks, this is called as multiplicity. The most common concept associated with signal multiplicity is the n+1 rule. According to this rule, the signal for the proton under observation will be split into n+1 peaks by protons attached to adjacent carbons, where n is the number of such protons.
No. of peaks = n + 1
(n = no. of neighbouring chemically equivalent Hydrogen nuclii)
So, Hb —-> 4Ha
Multiplicity —->n + 1 = 5 (Quintet)
And, according to Pascal triangle(shown below): Intensity–> 1 : 4 : 6 : 4 : 1
Q.6. Which of the following organic compound with molecular formula C3H C12 exhibits only one signal in the IH NMR spectrum?
(a) 2, 2-dichloropropane
(b) 1, 2-dichloropropane
(c) 1, 3-dichloropropane
(d) 1, 1-dichloropropane
Correct Answer is Option (a)
Only (a) compound has all chemically equivalent hydrogen. So, give only one peak.
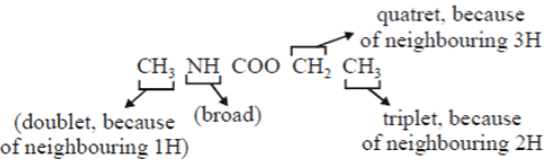
Q.7. Which of the following 1H-NMR spectrum of compound with molecular formula C4H9NO2 shows delta 5.30 (broad, IH), 4.10(q, 2H), 2.80 (d, 3H), 1.20 (t, 3H) ppm?
(a) CH3NHCOOCH2CH3
(b) CH3CH2NHCOOCH3
(c) CH3OCH2CONHCH3
(d) CH3CH2OCH2CONH2
Correct Answer is Option (a)
C4H9NO2;chemical shift = 5.30 (broad, 1H)
chemical shift = 4.10 (q, 2H)
chemical shift = 2.80 (d, 3H)
chemical shift = 1.20 (t, 3H)
As shown in the above compound, doublet, because of neighbouring 1H attached to N and one will be broad as because of hydrogen attached to N, quatret becauseof neighbouring 3H and finally triplet, becauseof neighbouring 2H.
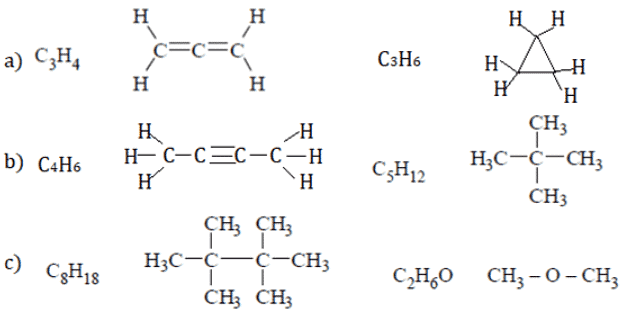
Q.8. Which of the compound show only one signal is present in the PMR spectra?
(a) C3H4, C3H6
(b) C4H6, C5H12
(c) C8H18, C2H6O
(d) All of the mentioned
Correct Answer is Option (d)
All of the below compounds have only type of proton, so only one signal.
Q.9. Compound C4H10O gave PMR spectrum consisting of two groups of lines (multiplets) with relative intensities in the ratio 3 : 2. Other compound of the same formula exhibited two lines with relative area of 9 : 1. What are these compounds.
(a) Diethyl ether and n-butanol
(b) t-Butyl alcohol and 1-Methoxypropane
(c) diethyl ether and t-Butyl alcohol
(d) Diethyl ether and 2-Methoxypropane
Correct Answer is Option (c)
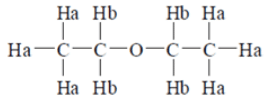
NMR data: Two groups of lines in 3 : 2 and two groups of lines in 9 : 1 for same molecular formula(a) CH3–CH2–O–CH2–CH3
6Ha -> 1 group of line;
4Hb -> 1 group of line
Therefore, Intensity => 6 : 4 => 3 : 2.
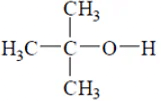
(b)
9H -> 1 group of line and 1H -> 1 group of line / single peak.
Therefore, Intensity => 9 : 1.
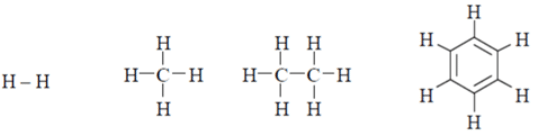
Q.10. H2, CH4, C2H6 and C6H6 exhibit which PMR spectra?
(a) Singlet
(b) Doublet
(c) Triplet
(d) Quintet
Correct Answer is Option (a)
All structures have only one type of hydrogen. So, all give single peak.
|
44 videos|102 docs|52 tests
|
FAQs on Solved Practice Questions on NMR Spectroscopy - Organic Chemistry
| 1. What is NMR spectroscopy? |  |
| 2. How does NMR spectroscopy work? |  |
| 3. What information can be obtained from NMR spectra? |  |
| 4. How is NMR spectroscopy used in organic chemistry? |  |
| 5. What are the advantages of NMR spectroscopy? |  |