Solved Practice Questions: Electrophiles and Nucleophiles | Organic Chemistry PDF Download
Q.1. In the given molecule where will electrophile will attack?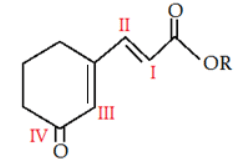 (a) I
(a) I
(b) II
(c) III
(d) IV
Correct Answer is Option (a)
-OCOR is a +M group, it is more electron rich than rest of the groups, so it has lone pair to donate to upcoming electrophile.
Q.2. Which reagent is a good nucleophile?
(a) NH3
(b) BH3
(c) Br2
(d) HBr
Correct Answer is Option (a)
Less electronegative atoms make better nucleophiles as they are more willing to share electrons.
Q.3. Which of the following is most readily undergo electrophilic attack?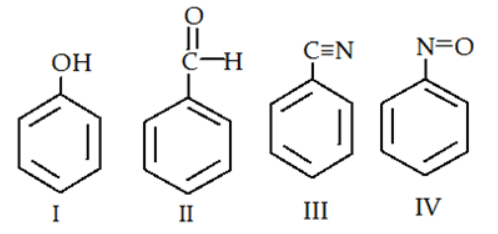 (a) I
(a) I
(b) II
(c) III
(d) IV
Correct Answer is Option (a)
I is most reactive towards electrophilic attack, as -OH is +M group therefore, here benzene will have more e- density than rest of the others.
Q.4. Which of the following statements is true about ammonia and water?
(a) ammonia is more basic and more nucleophilic than water
(b) ammonia is less basic and less nucleophilic than water
(c) ammonia is more basic but less nucleophilic than water
(d) ammonia is less basic but more nucleophilic than water
Correct Answer is Option (a)
Because oxygen is more electronegative than nitrogen, it holds onto its lone pairs more tightly than nitrogen, and hence is less likely to donate its lone pairs to form a covalent bond with a carbon atom during a nucleophilic attack.
Q.5. What is the correct order for the rate of reaction for the electrophilic attack of the given compounds?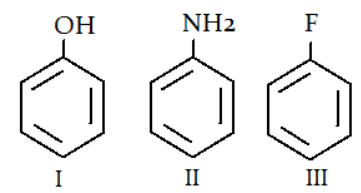 (a) I > II > III
(a) I > II > III
(b) II > I > III
(c) I < II < III
(d) III > I > II
Correct Answer is Option (b)
Electronegativity trend is N < O < F, so III is the least electron rich ring therefore least favourable for electrophilic attack. Similarly, II is the most electron rich ring therefore most favourable for electrophilic attack.
Q.6. Which of the following statements is true about the following two anionic molecules?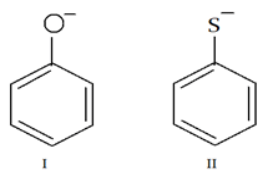 (a) I is more basic and more nucleophilic than II
(a) I is more basic and more nucleophilic than II
(b) I is less basic and less nucleophilic than II
(c) I is more basic but less nucleophilic than II
(d) I is less basic but more nucleophilic than II
Correct Answer is Option (c)
Larger atoms make better nucleophiles due to polarizability so II containing S will be more nucleophilic than I but less basic.
Q.7. Which of the following is the product for the given reaction?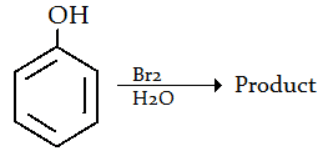 (a) 3-Bromophenol
(a) 3-Bromophenol
(b) 4-Bromophenol
(c) 3, 5-Dibromophenol
(d) 2, 4, 6-Tribromophenol
Correct Answer is Option (d)
The polar solvent (i.e. water) induces a stronger dipole in Br-Br. A stronger electrophile (Br+) is produced that undergoes electrophilic aromatic substitution with the benzene ring more readily. Consequently, you get tri-bromination with Br(aq.).
Q.8. Which halide ion is the best nucleophile in dimethyl sulfoxide solution?
(a) I–
(b) F–
(c) Cl–
(d) Br–
Correct Answer is Option (b)
In polar, aprotic solvents, F– strongest is the nucleophile, and I– the weakest.
Q.9. Which ring will readily undergo electrophilic attack?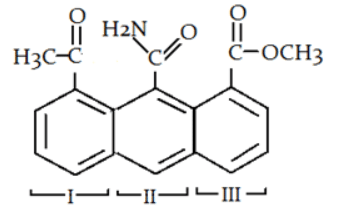 (a) I
(a) I
(b) II
(c) III
(d) Both I and III
Correct Answer is Option (b)
Due to the presence of -NH2 group electron will be transferred to the C of -CONH2 group and that’s why it will not withdraw electron from benzene ring. So, electron density of ring II is most in all three rings and that is why electrophile will attack at ring II.
Q.10. Which of the following cannot react as a nucleophile?
(a) CH3NH2
(b) (CH3)2NH
(c) (CH3)3N
(d) (CH3)4N+
Correct Answer is Option (d)
Nucleophile donates electron to the electrophiles and there is no lone pair present on nitrogen (CH3)4N+, so donation of electron is not possible.
|
39 videos|92 docs|46 tests
|
FAQs on Solved Practice Questions: Electrophiles and Nucleophiles - Organic Chemistry
| 1. What is an electrophile? |  |
| 2. What is a nucleophile? |  |
| 3. How do electrophiles and nucleophiles react with each other? |  |
| 4. What are some examples of electrophiles? |  |
| 5. What are some examples of nucleophiles? |  |
















