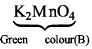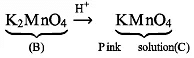Solved Practice Questions on Oxidation & coordination state of central metal ions | Inorganic Chemistry PDF Download
Q.1. Give the oxidation number and coordination number of the central metal atom in the complex compound K3[Fe(C2O4)3].
Let us consider the above coordination compound K3[Fe(C2O4)3]. Here iron is the central metal atom. Let the oxidation state of the central metal atom is x. Since Potassium is the alkali metal and we know the oxidation state of alkali metal is 1. C2O4 , i.e. oxalate ions act as a ligand. It is an anionic ligand and its denticity is 2 so the oxidation state of oxalate ion is 2.
As we know the oxidation state of the whole compound is equal to the sum of the oxidation state of all the atoms present in a compound.
So the oxidation state Fe is calculated as shown below:
+3 + x + 3 × (−2) = 0
⇒ 3 + x − 6 = 0
⇒ x − 3 = 0
⇒ x =3Hence the oxidation state of iron (Fe) is +3.
In the coordination compound, the coordination number is also defined as the number of ligands that are bonded to the central metal atom by the coordinate bond. Here three oxalate ion is coordinated with central metal atom i.e. Fe. Hence the coordination number is 3.
Note:
Alternatively we can also find the oxidation number as shown below:
The given coordination compound is K3[Fe(C2O4)3]. Since potassium carries a +1 charge and as the complex is neutral so the charge on complexion should be -3. Hence the complex ion will be written as [Fe(C2O4)3]−3. Let us take the oxidation state Fe is x. Oxalate ion is bidentate ligand hence, carries the charge -2. On adding the charge of all the atom we get the overall oxidation state as shown below:
x + 3 × (−2) = − 3
⇒ x − 6 = − 3
⇒ x = − 3 + 6
⇒ x = 3
Q.2. In the complex ion [Fe(EDTA)] − the coordination number and oxidation state of central metal ion is:
(a) C.N. = 6 O.N. = +3
(b) C.N. = 1 O.N. = -1
(c) C.N. = 4 O.N. = +2
(d) C.N. = 3 O.N. = +3
Correct Answer is option (a)
In [Fe(EDTA)] −, the denticity of EDTA=6, so the co-ordination number =6.
The change of EDTA=−4
Let oxidation state of Fe is n.
⇒n−4=−1
n=+3
⇒C.N=6; Oxidation number=+3
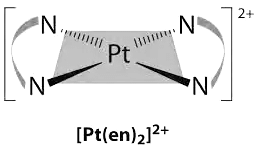
Q.3. The coordination number and oxidation number of the central metal ion in the complex [Pt(en)2]+2 is:
(a) C.N. = 2 O.N. = +2
(b) C.N. = 6 O.N. = +4
(c) C.N. = 4 O.N. = +4
(d) C.N. = 4 O.N. = +2
Correct Answer is option (d)
Ethylenediamine which is bidentate ligand is of two in number hence 2X2 =4
Hence co-ordination number of Platinum will be 4
Oxidation state = [x + 2 (0)] = 2+
x =+2
Thus, coordination number is 4 and oxidation number is +2
Q.4. The oxidation states of central metal ions of (A), (B) and (C) compounds are respectively:
(a) +II, +VI and + VII
(b) +II, + VI and + VI
(c) +II, + VII and + VII
(d) +VI, + VII and +VII
Correct Answer is option (a)
Mn(N03)2 + (K2C03 +KN03) →
+ 4NaOH + Br2 → 2NaBr +
+ 2NaNO3 + 2H2O
MnO2 + PbO2 + HNO3 →
+ Pb(NO3)2 + 2H2O
Mn(NO3)2 + BaCl2 → Ba(NO3)2 +
Oxidation state of A : Mn(NO3)2 → +2
Oxidation state of B: K2MnO4 → +6
Oxidation state of C: K M nO4 → +7
|
48 videos|92 docs|41 tests
|
FAQs on Solved Practice Questions on Oxidation & coordination state of central metal ions - Inorganic Chemistry
| 1. What is oxidation? |  |
| 2. What is meant by the coordination state of central metal ions? |  |
| 3. How does oxidation affect the coordination state of central metal ions? |  |
| 4. Are there any specific rules or guidelines to determine the oxidation state of central metal ions? |  |
| 5. Can the coordination state of central metal ions affect the reactivity of a coordination compound? |  |