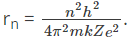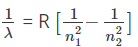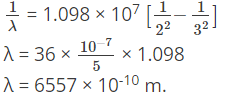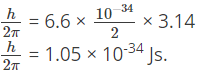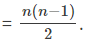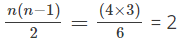Solved Practice Questions on Developments Leading to the Bohr’s Model of Atom | Physical Chemistry PDF Download
Q.1. The radius of the Bohr orbit depends on which of the following?
(a) 1/n
(b) n
(c) 1/n2
(d) n2
Correct Answer is Option (c)
The equation is given as:
From this, we can understand that rn is directly proportional to n2.
Q.2. What’s the wavelength for the visible region in electromagnetic radiation?
(a) 400 – 750 nm
(b) 400 – 750 mm
(c) 400 – 750 μm
(d) 400 – 750 pm
Correct Answer is Option (a)
Electromagnetic spectrum is made up of various electromagnetic radiation. They are radio waves, X-rays, gamma rays, UV rays, the visible region, IR waves, and microwaves. Visible rays are the only ones which a human eye can see. They range from 450 – 750 nm.
Q.3. What is the order of the radius of an electron orbit in a hydrogen atom?
(a) 10-8 m
(b) 10-9 m
(c) 10-11 m
(d) 10-13 m
Correct Answer is Option (c)
The radius of an electron orbit in a hydrogen atom is of the order of 10-11 m. It is equal to the most probable distance between the nucleus and the electron in a hydrogen atom in its ground state.
Q.4. What is the wavenumber of violet color?
(a) 25 x 103 mm-1
(b) 25 x 103 m-1
(c) 25 x 103 cm-1
(d) 25 x 103 nm-1
Correct Answer is Option (c)
The wavenumber is the reciprocal or the inverse of wavelength. Wavenumber = 1/Wavelength. Its unit is cm-1. The wavelength of violet color is 400nm as seen in the electromagnetic spectrum. So wavenumber = 1/400nm = 25 x 103 cm-1.
Q.5. What will be the longest wavelength in the Balmer series of hydrogen spectrum?
(a) 6557 × 10-10 m
(b) 5557 × 10-10 m
(c) 9557 × 10-10 m
(d) 1557 × 10-10 m
Correct Answer is Option (a)
Q.6. Calculate the frequency of the wave whose wavelength is 10nm.
(a) 2 Hz
(b) 3 Hz
(c) 1 Hz
(d) 4 Hz
Correct Answer is Option (b)
The relation between wavelength(λ) and frequency(v) of a wave is given by λ = c/v where c is the speed of light of the light. v = c/λ Frequency of the given wave = (3 x 108m/s)/(10 x 10-9m) = 3 Hz.
Q.7. A hydrogen atom in its ground state absorbs 10.2 eV of energy. What is the orbital angular momentum is increased by?
(a) 4.22 × 10-3 Js
(b) 2.11 × 10-34 Js
(c) 3.16 × 10-34 Js
(d) 1.05 × 10-34 Js
Correct Answer is Option (d)
Increase in angular momentum = h/2π.
Q.8. If Energy = 4.5 KJ; calculate the wavelength.
(a) 4.42 x 10-29 m
(b) 4.42 x 10-39 m
(c) 4.42 x 10-25 m
(d) 4.42 x 10-22 m
Correct Answer is Option (a)
We know E = hv through Planck’s Quantum Theory, where E is energy, h is Planck’s constant and v is the frequency. 4.5 KJ = (6.626×10–34 Js)(3 x 108m/s)/(wavelength). wavelength = 4.42 x 10-29 m.
Q.9. Which of the following is true regarding the Bohr model of atoms?
(a) Assumes that the angular momentum of electrons is quantized
(b) Uses Faraday’s laws
(c) Predicts continuous emission spectra for atoms
(d) Predicts the same emission spectra for all types of atoms
Correct Answer is Option (a)
Bohr model of atoms assumes that the angular momentum of electrons is quantized. The atom is held between the nucleus and surroundings by electrostatic forces. The other options are not valid.
Q.10. ________ frequency, is the minimum frequency required to eject an electron when photons hit the metal surface.
(a) Required
(b) Activated
(c) Threshold
(d) Limiting
Correct Answer is Option (c)
In the photoelectric effect, when photons strike on a metal surface, it emits electrons. Thus for emitting an electron, it requires a minimum amount of energy. This is threshold energy acquired through threshold frequency.
Q.11. In terms of Bohr radius a0, the radius of the second Bohr orbit of a hydrogen atom is given by √2 a0.
(a) True
(b) False
Correct Answer is Option (b)
The equation is given as:
rn = r1 n2
r2 = (2)2r0 = 4r0. Thus, in terms of Bohr radius a0, the radius of the second Bohr orbit of a hydrogen atom is given by 4 a0.
Q.12. A metal’s work function is 3.8KJ. Photons strike metal’s surface with an energy of 5.2 KJ. what’s the kinetic energy of the emitted electrons?
(a) 3.8 KJ
(b) 5.2 KJ
(c) 9 KJ
(d) 1.4 KJ
Correct Answer is Option (d)
As per the formula of the photoelectric effect, we have E = K.E. + Wo. E is the energy of photons; K.E. is the kinetic energy with which electrons are emitted and Wo is the work function. K.E. = 5.2 KJ – 3.8 KJ = 1.4 KJ.
Q.13. Hydrogen atoms are excited from ground state to the state of principal quantum number 4. Then, what will be the number of spectral lines observed?
(a) 3
(b) 6
(c) 5
(d) 2
Correct Answer is Option (d)
n = 4.
The number of spectral lines emitted
Q.14. When an electron jumps from 3rd orbit to 2nd orbit, which series of spectral lines are obtained?
(a) Balmer
(b) Lyman
(c) Paschen
(d) Brackett
Correct Answer is Option (a)
As per the spectral lines of the hydrogen, when an electron jumps from nth orbit to 2nd orbit, it’s in Balmer series (provided that n = 3, 4, 5….). For Balmer series, the electron emits waves in visible region.
Q.15. When a hydrogen atom is in its first excited level, what is the relation of radius and Bohr radius?
(a) Twice
(b) 4 times
(c) Same
(d) Half
Correct Answer is Option (b)
For the first excited level, n = 2.
r2 = (2)2r0 = 4r0.
So, when a hydrogen atom is in its first excited level, its radius is 4 times of the Bohr radius.
Q.16. Find out the wavenumber, when an electron jumps from 2nd orbit to 1st.
(a) 82357.75 cm-1
(b) 105,677 cm-1
(c) 82257.75 cm-1
(d) 109,677 cm-1
Correct Answer is Option (c)
The Swedish spectroscopist, Johannes Rydberg gave a formula; Wavenumber = RH[(1/n1)2-(1/n2)2]. Here RH is the Rydberg constant and is equal to 109,677 cm-1. Wavenumber = 109,677(3/4) = 82257.75 cm-1.
Q.17. The ultraviolet spectral region is obtained in Balmer series.
(a) True
(b) False
Correct Answer is Option (b)
When an electron jumps from nth orbit to 1st orbit, provided that n = 1, 2, 3, etc, it emits radion in the ultraviolet region. As per the spectral lines of the hydrogen, when an electron jumps from nth orbit to 2nd orbit, it’s in Balmer series (provided that n = 3, 4, 5….). For Balmer series, the electron emits waves in the visible region.
Q.18. During the photoelectric effect, when photons strike with 5.1eV, electrons emitted from which metal have higher kinetic energy?
 (a) Na
(a) Na
(b) Ag
(c) Equal
(d) Neither
Correct Answer is Option (a)
As per the formula of the photoelectric effect, we have E = K.E. + Wo. E is the energy of photons; K.E. is the kinetic energy with which electrons are emitted and Wo is the work function. K.E. of Na and Ag are 2.8 eV and 0.8 eV.
|
83 videos|142 docs|67 tests
|