JAMB Exam > JAMB Notes > Chemistry for JAMB > Separation Techniques
Separation Techniques | Chemistry for JAMB PDF Download
Introduction
- This is used to separate a liquid and soluble solid from a solution (e.g., water from a solution of salt water) or a pure liquid from a mixture of liquids
- The solution is heated, and pure water evaporates producing a vapour which rises through the neck of the round bottomed flask
- The vapour passes through the condenser, where it cools and condenses, turning into the pure liquid that is collected in a beaker
- After all the water is evaporated from the solution, only the solid solute will be left behind
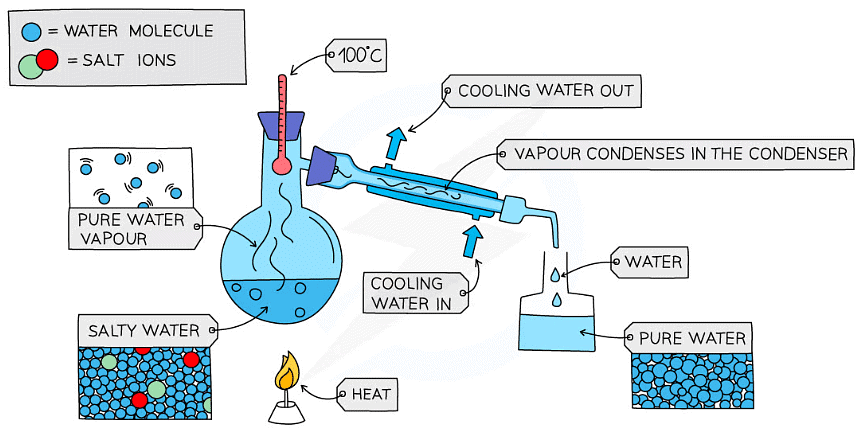 Diagram showing the distillation of a mixture of salt and water
Diagram showing the distillation of a mixture of salt and water
Fractional Distillation
- This is used to separate two or more liquids that are miscible with one another (e.g., ethanol and water from a mixture of the two)
- The solution is heated to the temperature of the substance with the lowest boiling point
- This substance will rise and evaporate first, and vapours will pass through a condenser, where they cool and condense, turning into a liquid that will be collected in a beaker
- All of the substance is evaporated and collected, leaving behind the other components(s) of the mixture
- For water and ethanol
- Ethanol has a boiling point of 78 ºC and water of 100 ºC
- The mixture is heated until it reaches 78 ºC, at which point the ethanol boils and distills out of the mixture and condenses into the beaker
- When the temperature starts to increase to 100 ºC heating should be stopped. Water and ethanol are now separated
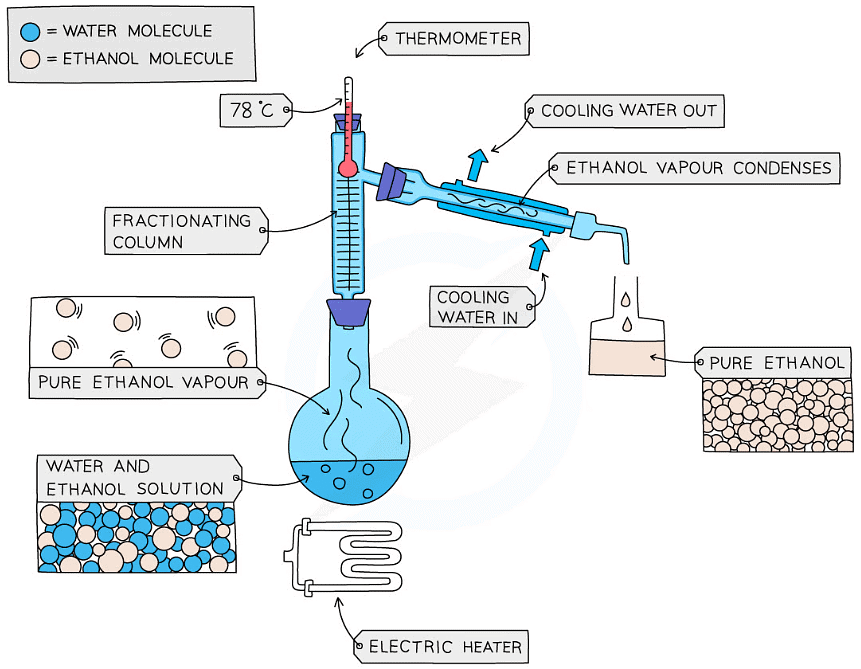 Fractional distillation of a mixture of ethanol and water
Fractional distillation of a mixture of ethanol and water
Filtration
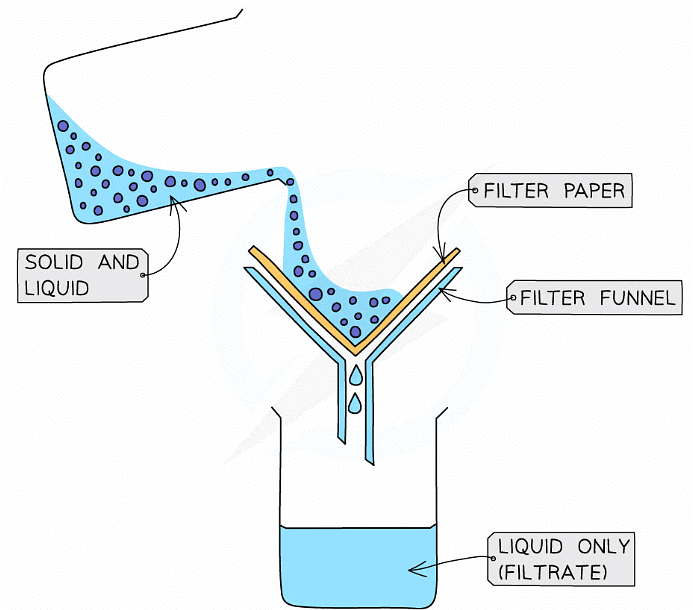
- Used to separate an undissolved solid from a mixture of the solid and a liquid / solution ( e.g., sand from a mixture of sand and water)
- Centrifugation can also be used for this mixture
- A piece of filter paper is placed in a filter funnel above a beaker
- A mixture of insoluble solid and liquid is poured into the filter funnel
- The filter paper will only allow small liquid particles to pass through as filtrate
- Solid particles are too large to pass through the filter paper so will stay behind as a residue
Crystallisation
- Used to separate a dissolved solid from a solution, when the solid is much more soluble in hot solvent than in cold (e.g., copper sulphate from a solution of copper (II) sulphate in water)
- The solution is heated, allowing the solvent to evaporate, leaving a saturated solution behind
- Test if the solution is saturated by dipping a clean, dry, cold glass rod into the solution
- If the solution is saturated, crystals will form on the glass rod
- The saturated solution is allowed to cool slowly
- Crystals begin to grow as solids will come out of solution due to decreasing solubility
- The crystals are collected by filtering the solution, they are washed with cold distilled water to remove impurities and are then allowed to dry
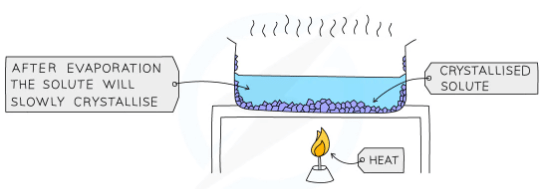 Diagram showing the process of crystallisation
Diagram showing the process of crystallisation
Paper Chromatography
- This technique is used to separate substances that have different solubilities in a given solvent (e.g., different coloured inks that have been mixed to make black ink)
- A pencil line is drawn on chromatography paper and spots of the sample are placed on it. Pencil is used for this as ink would run into the chromatogram along with the samples
- The paper is then lowered into the solvent container, making sure that the pencil line sits above the level of the solvent, so the samples don’t wash into the solvent container
- The paper is called the stationary phase
- The solvent travels up the paper by capillary action, taking some of the coloured substances with it; it is called the mobile phase
- Different substances have different solubilities so will travel at different rates, causing the substances to spread apart
- Those substances with higher solubility will travel further than the others
- This will show the different components of the ink / dye
- If two or more substances are the same, they will produce identical chromatograms
- If the substance is a mixture, it will separate on the paper to show all the different components as separate spots
- An impure substance will show up with more than one spot, a pure substance should only show up with one spot
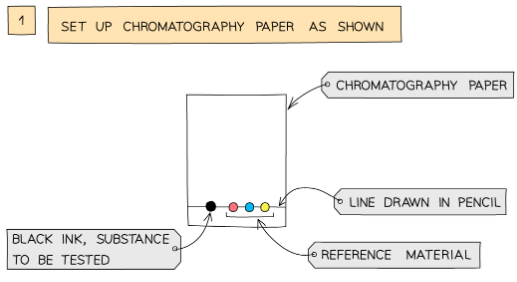
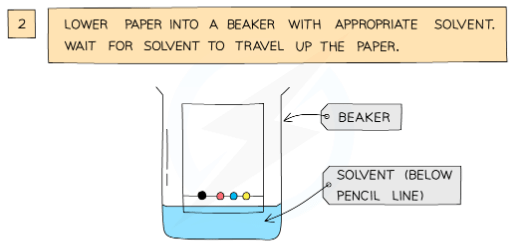
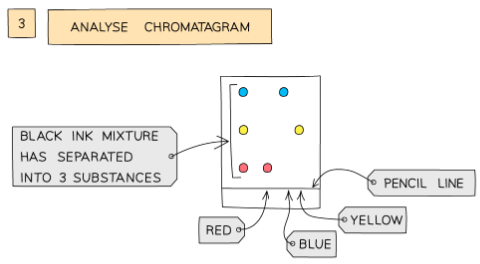 Analysis of the composition of ink using paper chromatography
Analysis of the composition of ink using paper chromatography
The document Separation Techniques | Chemistry for JAMB is a part of the JAMB Course Chemistry for JAMB.
All you need of JAMB at this link: JAMB
|
215 videos|220 docs|162 tests
|
Related Searches

















