Atomic Structure | Chemistry for Grade 12 PDF Download
Structure of an Atom
- All matter is composed of atoms, which are the smallest parts of an element that can take place in chemical reactions
- Atoms are mostly made up of empty space around a very small, dense nucleus that contains protons and neutrons
- The nucleus has an overall positive charge
- The protons have a positive charge and the neutrons have a neutral charge
- Negatively charged electrons are found in orbitals in the empty space around the nucleus
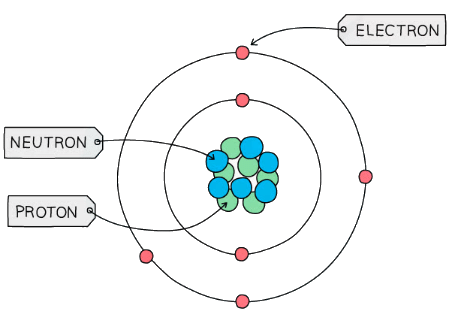 The basic structure of an atom (not to scale)
The basic structure of an atom (not to scale)
Subatomic Particles
- The protons, neutrons and electrons that an atom is made up of are called subatomic particles
- These subatomic particles are so small that it is not possible to measure their masses and charges using conventional units (such as grams or coulombs)
- Instead, their masses and charges are compared to each other, and so are called ‘relative atomic masses’ and ‘relative atomic charges’
- These are not actual charges and masses but charges and masses of particles relative to each other
- Protons and neutrons have a very similar mass, so each is assigned a relative mass of 1
- Electrons are 1836 times smaller than a proton and neutron, and so their mass is often described as being negligible
- The relative mass and charge of the subatomic particles are:
Relative mass & charge of subatomic particles table
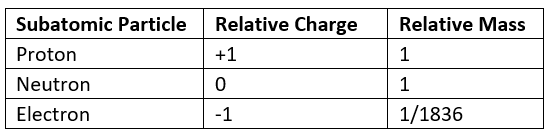
Atoms: Key Terms
- The atomic number (or proton number) is the number of protons in the nucleus of an atom and has the symbol Z
- The atomic number is also equal to the number of electrons present in a neutral atom of an element
- E.g. the atomic number of lithium is 3, meaning that a neutral lithium atom has 3 protons and therefore, also has 3 electrons
- The mass number (or nucleon number) is the total number of protons + neutrons in the nucleus of an atom, and has the symbol A
- The number of neutrons can be calculated by:
Number of neutrons = mass number - atomic number - Protons and neutrons are also called nucleons, because they are found in the nucleus
Mass & Charge Distribution
- The mass of an atom is concentrated in the nucleus, because the nucleus contains the heaviest subatomic particles (the neutrons and protons)
- The mass of the electron is negligible
- The nucleus is also positively charged due to the protons
- Electrons orbit the nucleus of the atom, contributing very little to its overall mass, but creating a ‘cloud’ of negative charge
- The electrostatic attraction between the positive nucleus and negatively charged electrons orbiting around it is what holds an atom together
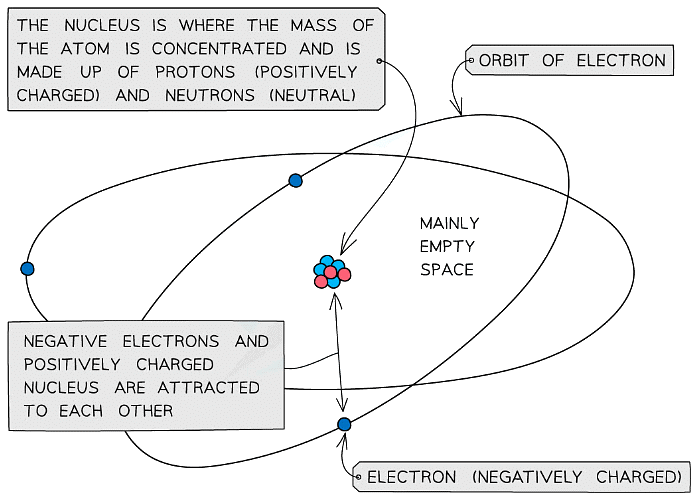 The mass of the atom is concentrated in the positively charged nucleus which is attracted to the negatively charged electrons orbiting around it
The mass of the atom is concentrated in the positively charged nucleus which is attracted to the negatively charged electrons orbiting around it
Behaviour of Subatomic Particles in an Electric Field
- Protons, neutrons and electrons behave differently when they move at the same velocity in an electric field
- When a beam of electrons is fired past the electrically charged plates, the electrons are deflected very easily away from the negative plate towards the positive plate
- This proves that the electrons are negatively charged; like charges repel each other
- It also shows that electrons have a very small mass, as they are easily deflected
- A beam of protons is deflected away from the positive plate and towards the negative plate
- This proves that the proton is positively charged
- As protons are deflected less than electrons, this also shows that protons are heavier than electrons
- A beam of neutrons is not deflected at all
- Which proves that the particle is neutral in character; it is not attracted to, or repelled by, the negative or positive plate
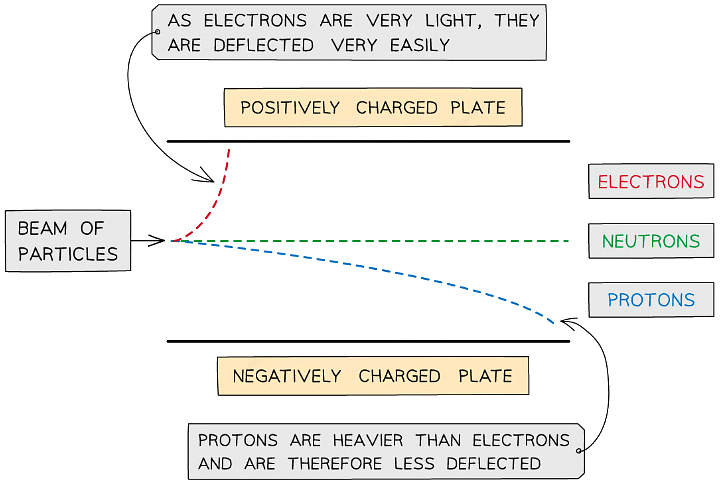 The lighter electrons undergo much more deflection than the protons
The lighter electrons undergo much more deflection than the protons
Determining the Subatomic Structure of Atoms & Ions
- An atom is neutral and has no overall charge
- Ions on the other hand are formed when atoms either gain or lose electrons, causing them to become charged
- The number of subatomic particles in atoms and ions can be determined given their atomic (proton) number, mass (nucleon) number and charge
Protons
- The atomic number of an atom and ion determines which element it is
- Therefore, all atoms and ions of the same element have the same number of protons (atomic number) in the nucleus
- E.g. lithium has an atomic number of 3 (three protons) whereas beryllium has atomic number of 4 (4 protons)
- The number of protons equals the atomic (proton) number
- The number of protons of an unknown element can be calculated by using its mass number and number of neutrons:
Mass number = number of protons + number of neutrons
Number of protons = mass number - number of neutrons
Example: Determine the Number of Protons
Determine the number of protons of the following ions and atoms:
1. Mg2+ ion
2. Carbon atom
3. An unknown atom of element X with mass number 63 and 34 neutrons
Answer 1: The atomic number of a magnesium atom is 12 suggesting that the number of protons in the magnesium element is 12
Therefore the number of protons in a Mg2+ ion is also 12
Answer 2: The atomic number of a carbon atom is 6 suggesting that a carbon atom has 6 protons in its nucleus
Answer 3: Use the formula to calculate the number of protons
Number of protons = mass number - number of neutrons
Number of protons = 63 - 34
Number of protons = 29
Element X is therefore copper
Electrons
- An atom is neutral and therefore has the same number of protons and electrons
- Ions have a different number of electrons to their atomic number depending on their charge
- A positively charged ion has lost electrons and therefore has fewer electrons than protons
- A negatively charged ion has gained electrons and therefore has more electrons than protons
Example: Determine the Number of Electrons
1. Mg2+ ion
2. Carbon atom
3. An unknown atom of element X with mass number 63 and 34 neutrons
Answer 1: The atomic number of a magnesium atom is 12 suggesting that the number of protons in the neutral magnesium atom is 12
- However, the 2+ charge in Mg2+ ion suggests it has lost two electrons
- It only has 10 electrons left now
Answer 2: The atomic number of a carbon atom is 6 suggesting that the neutral carbon atom has 6 electrons orbiting around the nucleus
Answer 3: The number of protons of element X can be calculated by:
Number of protons = mass number - number of neutrons
Number of protons = 63 - 34
Number of protons = 29
The neutral atom of element X therefore also has 29 electrons
Neutrons
- The mass and atomic numbers can be used to find the number of neutrons in ions and atoms:
Number of neutrons = mass number (A) - number of protons (Z)
Example: Determine the Number of Neutrons
Determine the number of neutrons of the following ions and atoms:
1. Mg2+ ion
2. Carbon atom
3. An unknown atom of element X with mass number 63 and 29 protons
Answer 1: The atomic number of a magnesium atom is 12 and its mass number is 24
Number of neutrons = mass number (A) - number of protons (Z)
Number of neutrons = 24 - 12
Number of neutrons = 12
The Mg2+ ion has 12 neutrons in its nucleus
Answer 2: The atomic number of a carbon atom is 6 and its mass number is 12
Number of neutrons = mass number (A) - number of protons (Z)
Number of neutrons = 12 - 6
Number of neutrons = 6
The carbon atom has 6 neutrons in its nucleus
Answer 3: The atomic number of an element X atom is 29 and its mass number is 63
Number of neutrons = mass number (A) - number of protons (Z)
Number of neutrons = 63 - 29
Number of neutrons = 34
The neutral atom of element X has 34 neutrons in its nucleus
Atomic & Ionic Radius
Atomic radius
- The atomic radius of an element is a measure of the size of an atom
- It is half the distance between the two nuclei of two covalently bonded atoms of the same type
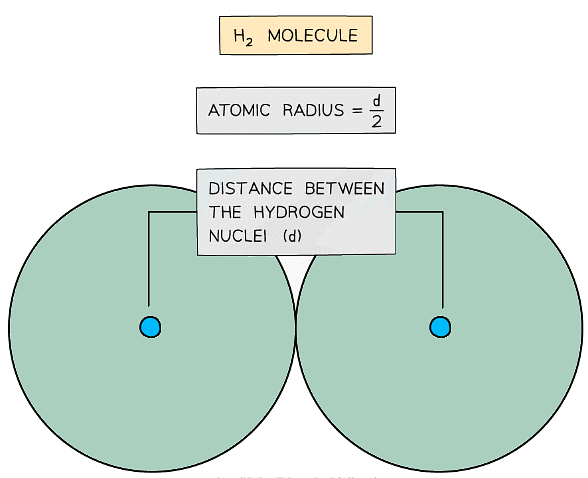 The atomic radius of a hydrogen atom is determined by halving the distance between the nuclei of two hydrogen atoms covalently bonded
The atomic radius of a hydrogen atom is determined by halving the distance between the nuclei of two hydrogen atoms covalently bonded
Atomic radii show predictable patterns across the Periodic Table
- They generally decrease across each Period
- They generally increase down each Group
These trends can be explained by the electron shell theory
- Atomic radii decrease as you move across a Period as the atomic number increases (increased positive nuclear charge) but at the same time extra electrons are added to the same principal quantum shell
- The larger the nuclear charge, the greater the pull of the nuclei on the electrons which results in smaller atoms
- Atomic radii increase moving down a Group as there is an increased number of shells going down the Group
- The electrons in the inner shells repel the electrons in the outermost shells, shielding them from the positive nuclear charge
- This weakens the pull of the nuclei on the electrons resulting in larger atoms
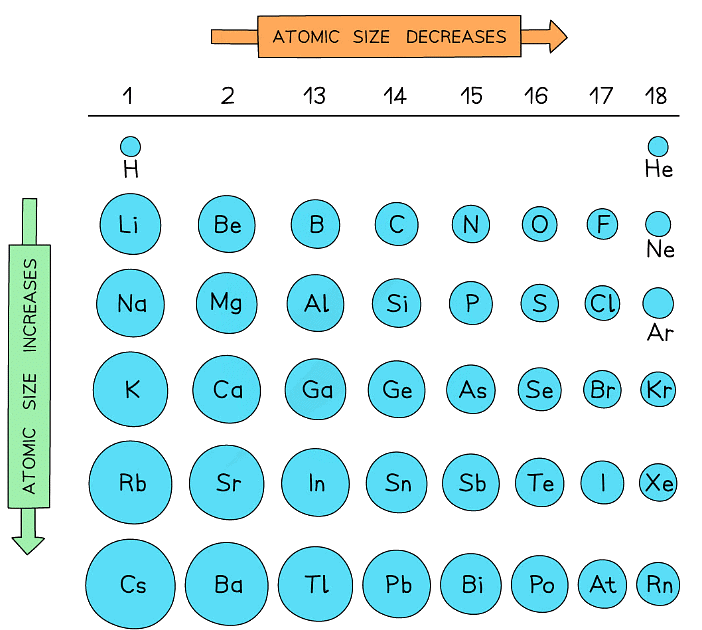 Trends in the atomic radii across a period and down a group
Trends in the atomic radii across a period and down a group
- The diagram shows that the atomic radius increases sharply between the noble gas at the end of each period and the alkali metal at the beginning of the next period
- This is because the alkali metals at the beginning of the next period have one extra principal quantum shell
- This increases shielding of the outermost electrons and therefore increases the atomic radius
Ionic radius
- The ionic radius of an element is a measure of the size of an ion
- Ionic radii show predictable patterns
- Ionic radii increase with increasing negative charge
- Ionic radii decrease with increasing positive charge
- These trends can also be explained by the electron shell theory
- Ions with negative charges are formed by atoms accepting extra electrons while the nuclear charge remains the same
- The outermost electrons are further away from the positively charged nucleus and are therefore held only weakly to the nucleus which increases the ionic radius
- The greater the negative charge, the larger the ionic radius
- Positively charged ions are formed by atoms losing electrons
- The nuclear charge remains the same but there are now fewer electrons which undergo a greater electrostatic force of attraction to the nucleus which decreases the ionic radius
- The greater the positive charger, the smaller the ionic radius
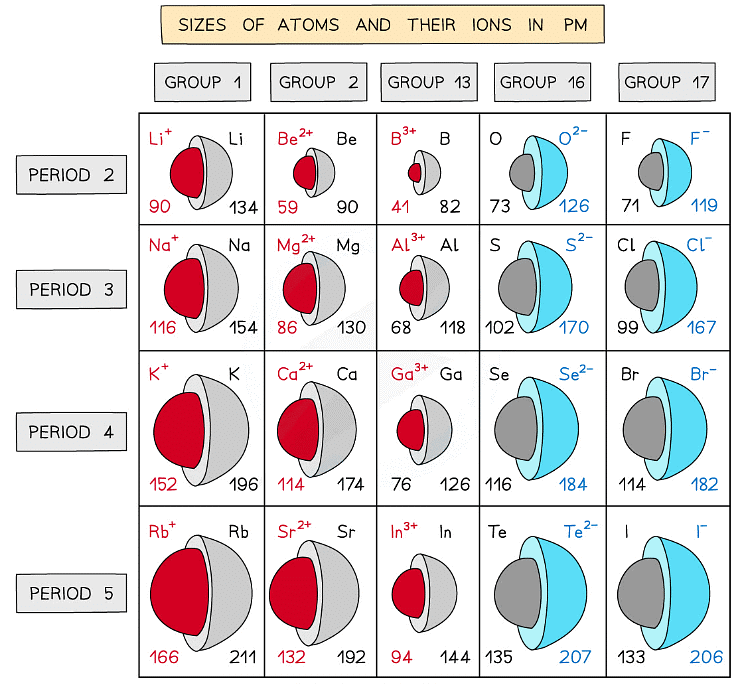 Trends in the ionic radii across a period and down a group
Trends in the ionic radii across a period and down a group
Isotopes: Basics
- Isotopes are atoms of the same element that contain the same number of protons and electrons but a different number of neutrons
- The symbol for an isotope is the chemical symbol (or word) followed by a dash and then the mass number
- Eg. carbon-12 and carbon-14 are isotopes of carbon containing 6 and 8 neutrons respectively
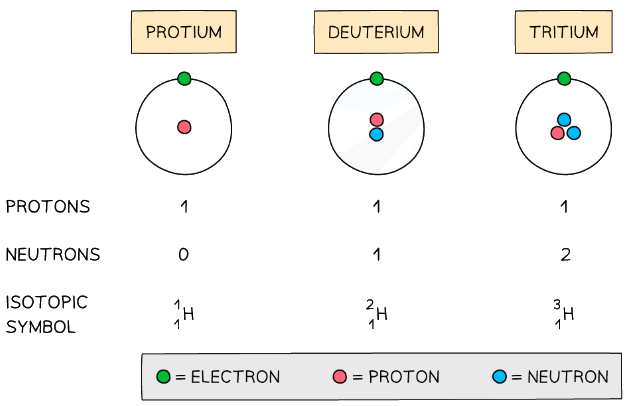 The atomic structure and symbols of the three isotopes of hydrogen
The atomic structure and symbols of the three isotopes of hydrogen
Isotopes: Chemical & Physical Properties
- Isotopes have similar chemical properties but different physical properties
Chemical properties
- Isotopes of the same element display the same chemical characteristics
- This is because they have the same number of electrons in their outer shells
- Electrons take part in chemical reactions and therefore determine the chemistry of an atom
Physical properties
- The only difference between isotopes is the number of neutrons
- Since these are neutral subatomic particles, they only add mass to the atom
- As a result of this, isotopes have different physical properties such as small differences in their mass and density
Electron Shells: Basics
Shells
- The arrangement of electrons in an atom is called the electronic configuration
- Electrons are arranged around the nucleus in principal energy levels or principal quantum shells
- Principal quantum numbers (n) are used to number the energy levels or quantum shells
- The lower the principal quantum number, the closer the shell is to the nucleus
- The higher the principal quantum number, the higher the energy of the shell
- Each principal quantum number has a fixed number of electrons it can hold
- n = 1 : up to 2 electrons
- n = 2 : up to 8 electrons
- n = 3 : up to 18 electrons
- n = 4 : up to 32 electrons
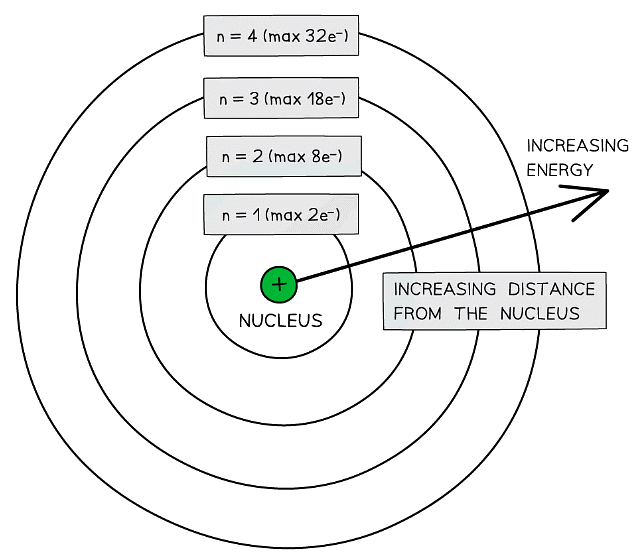 Electrons are arranged in principal quantum shells, which are numbered by principal quantum numbers
Electrons are arranged in principal quantum shells, which are numbered by principal quantum numbers
Subshells
- The principal quantum shells are split into subshells which are given the letters s, p and d
- Elements with more than 57 electrons also have an f shell
- The energy of the electrons in the subshells increases in the order s < p < d
- The order of subshells appear to overlap for the higher principal quantum shells as seen in the diagram below:
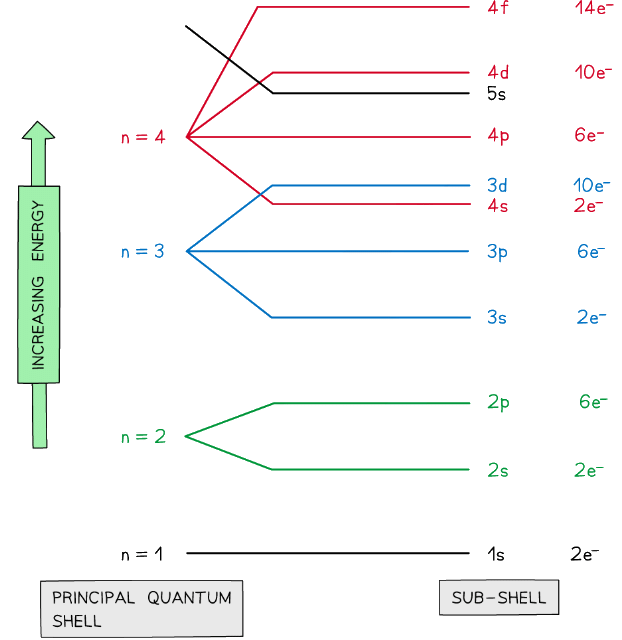 Electrons are arranged in principal quantum shells, which are numbered by principal quantum numbers
Electrons are arranged in principal quantum shells, which are numbered by principal quantum numbers
Orbitals
- Subshells contain one or more atomic orbitals
- Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them
- Each atomic orbital can be occupied by a maximum of two electrons
- This means that the number of orbitals in each subshell is as follows:
- s : one orbital (1 x 2 = total of 2 electrons)
- p : three orbitals ( 3 x 2 = total of 6 electrons)
- d : five orbitals (5 x 2 = total of 10 electrons)
- f : seven orbitals (7 x 2 = total of 14 electrons)
- The orbitals have specific 3-D shapes
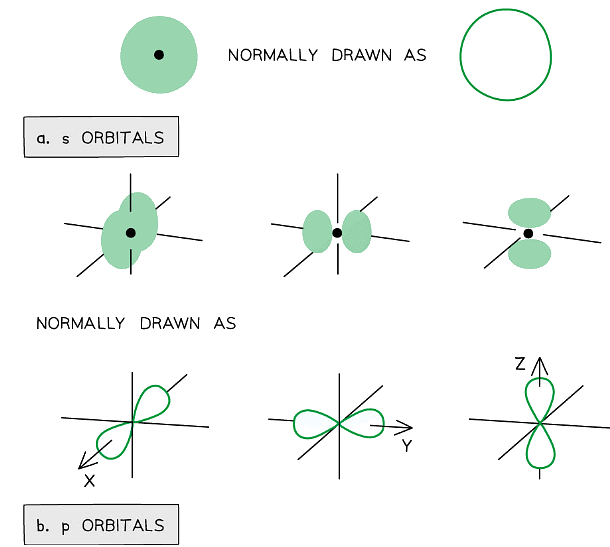 Representation of orbitals (the dot represents the nucleus of the atom) showing spherical s orbitals (a), p orbitals containing ‘lobes’ along the x, y and z axis
Representation of orbitals (the dot represents the nucleus of the atom) showing spherical s orbitals (a), p orbitals containing ‘lobes’ along the x, y and z axis
- Note that the shape of the d orbitals is not required at AS Level
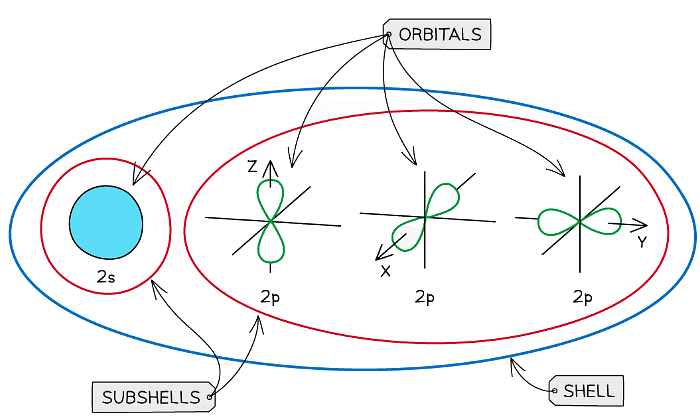 An overview of the shells, subshells and orbitals in an atom
An overview of the shells, subshells and orbitals in an atom
Ground state
- The ground state is the most stable electronic configuration of an atom which has the lowest amount of energy
- This is achieved by filling the subshells of energy with the lowest energy first (1s)
- The order of the subshells in terms of increasing energy does not follow a regular pattern at n= 3 and higher
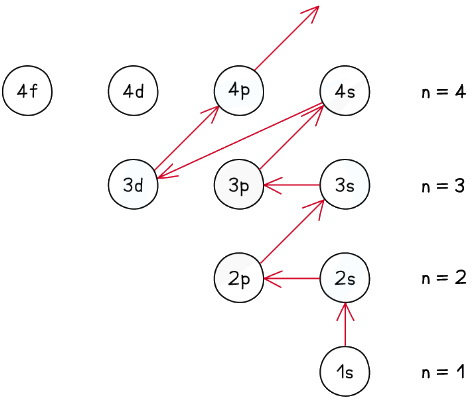 The ground state of an atom is achieved by filling the lowest energy subshells first
The ground state of an atom is achieved by filling the lowest energy subshells first
Electron Orbitals
- Each shell can be divided further into subshells, labelled s, p, d and f
- Each subshell can hold a specific number of orbitals:
- s subshell : 1 orbital
- p subshell : 3 orbitals labelled px, py and pz
- d subshell : 5 orbitals
- f subshell : 7 orbitals
- Each orbital can hold a maximum number of 2 electrons so the maximum number of electrons in each subshell are as follows:
- s : 1 x 2 = total of 2 electrons
- p : 3 x 2 = total of 6 electrons
- d : 5 x 2 = total of 10 electrons
f : 7 x 2 = total of 14 electrons
In the ground state, orbitals in the same subshell have the same energy and are said to be degenerate, so the energy of a px orbital is the same as a py orbital
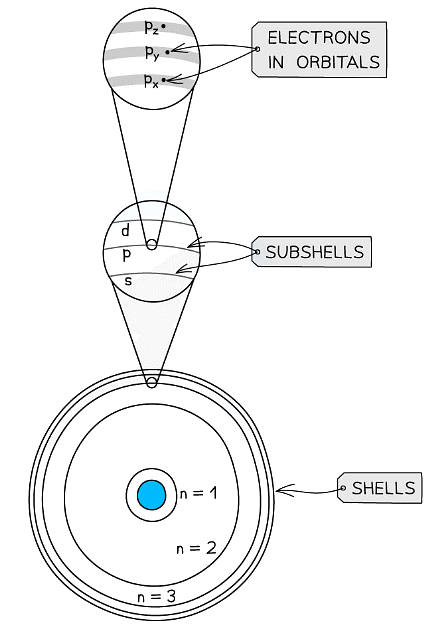 Shells are divided into subshells which are further divided into orbitals
Shells are divided into subshells which are further divided into orbitals
Summary of the arrangement of electrons in atoms table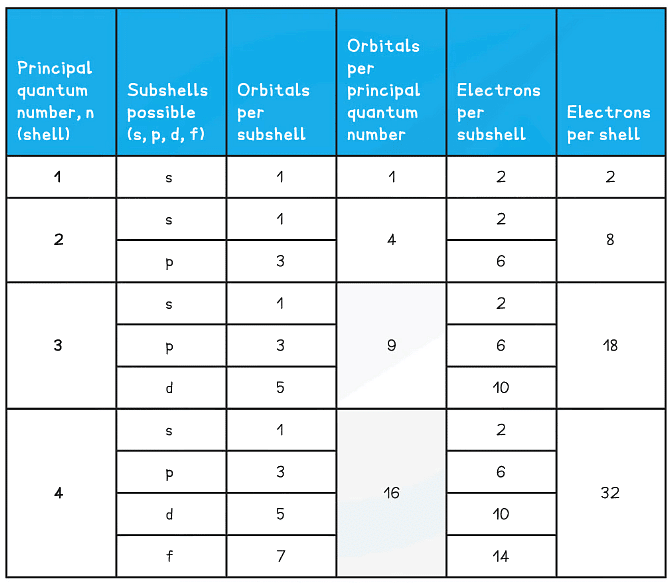
Subshells & Energy
- The principal quantum shells increase in energy with increasing principal quantum number
- E.g. n = 4 is higher in energy than n = 2
- The subshells increase in energy as follows: s < p < d < f
- The only exception to these rules is the 3d orbital which has slightly higher energy than the 4s orbital
- Because of this, the 4s orbital is filled before the 3d orbital
- All the orbitals in the same subshell have the same energy and are said to be degenerate
- E.g. px, py and pz are all equal in energy
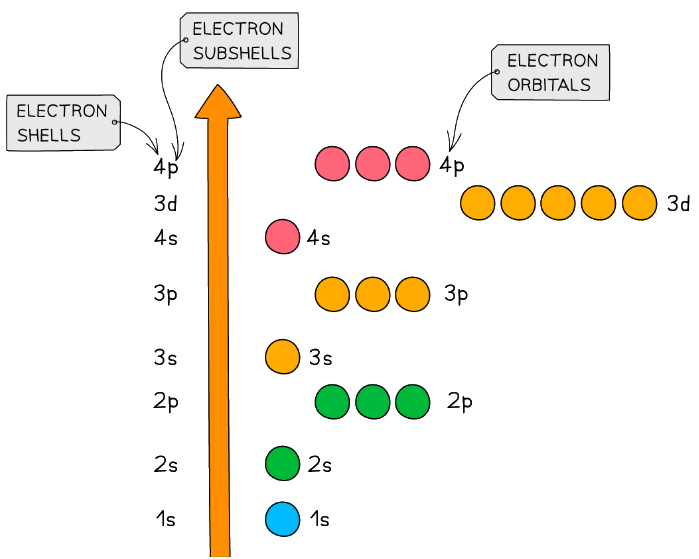 Relative energies of the shells and subshells
Relative energies of the shells and subshells
The s & p Orbitals
s orbitals
- The s orbitals are spherical in shape
- The size of the s orbitals increases with increasing shell number
- E.g. the s orbital of the third quantum shell (n = 3) is bigger than the s orbital of the first quantum shell (n = 1)
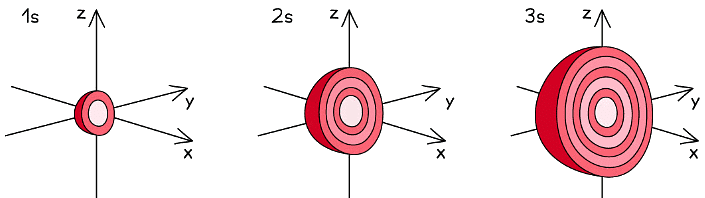 The s orbitals become larger with increasing principal quantum number
The s orbitals become larger with increasing principal quantum number
p orbitals
- The p orbitals are dumbbell-shaped
- Every shell has three p orbitals except for the first one (n = 1)
- The p orbitals occupy the x, y and z-axis and point at right angles to each other so are oriented perpendicular to one another
- The lobes of the p orbitals become larger and longer with increasing shell number
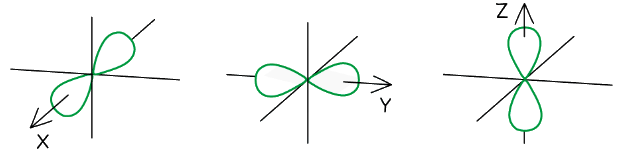 The p orbitals become larger and longer with increasing principal quantum number
The p orbitals become larger and longer with increasing principal quantum number
Electron Configurations: Basics
- The electron configuration gives information about the number of electrons in each shell, subshell and orbital of an atom
- The subshells are filled in order of increasing energy
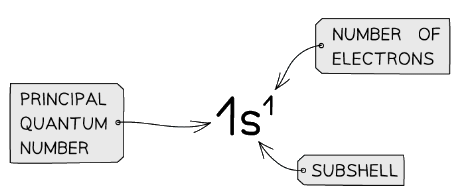 The electron configuration shows the number of electrons occupying a subshell in a specific shell
The electron configuration shows the number of electrons occupying a subshell in a specific shell
Electron Configurations: Explained
Electrons can be imagined as small spinning charges which rotate around their own axis in either a clockwise or anticlockwise direction
- The spin of the electron is represented by its direction
 Electrons can spin either in a clockwise or anticlockwise direction around their own axis
Electrons can spin either in a clockwise or anticlockwise direction around their own axis- Electrons with similar spin repel each other which is also called spin-pair repulsion
- Electrons will therefore occupy separate orbitals in the same subshell to minimize this repulsion and have their spin in the same direction
- Eg. if there are three electrons in a p subshell, one electron will go into each px, py and pz orbital
 Electron configuration: three electrons in a p subshell
Electron configuration: three electrons in a p subshell
- Electrons are only paired when there are no more empty orbitals available within a subshell in which case the spins are the opposite spins to minimize repulsion
- Eg. if there are four electrons in a p subshell, one p orbital contains 2 electrons with opposite spin and two orbitals contain one electron only
 Electron configuration: four electrons in a p subshell
Electron configuration: four electrons in a p subshell
- The principal quantum number indicates the energy level of a particular shell but also indicates the energy of the electrons in that shell
- A 2p electron is in the second shell and therefore has an energy corresponding to n = 2
- Even though there is repulsion between negatively charged electrons (inter-electrons repulsion), they occupy the same region of space in orbitals
- This is because the energy required to jump to successive empty orbital is greater than the inter-electron repulsion
- For this reason, they pair up and occupy the lower energy levels first
Electron Box Notation
- The electron configuration can also be represented using the electrons in boxes notation
- Each box represents an atomic orbital
- The boxes are arranged in order of increasing energy from lowest to highest
- The electrons are represented by opposite arrows to show the spin of the electrons
- Eg. the box notation for titanium is shown below
- Note that since the 3d subshell cannot be either full or half full, the second 4s electron is not promoted to the 3d level and stays in the 4s orbital
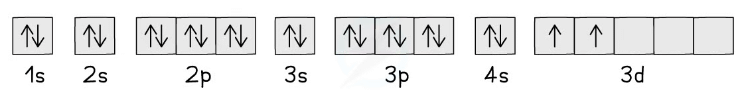 The electrons in Titanium are arranged in their orbitals as shown. Electrons occupy the lowest energy levels first before filling those with higher energy
The electrons in Titanium are arranged in their orbitals as shown. Electrons occupy the lowest energy levels first before filling those with higher energy
Free Radicals
- A free radical is a species with one or more unpaired electron
- The unpaired electron in the free radical is shown as a dot
- Eg. a chlorine free radical has the electron configuration 1s22s22p63s23p5
- Two of the three p orbitals have paired electrons whereas one of them has an unpaired electron
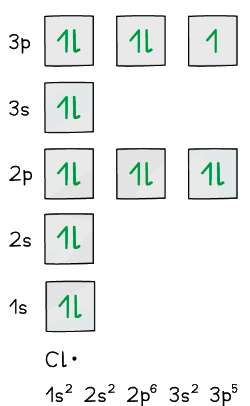 One of the p orbitals has unpaired electrons in a chlorine radical
One of the p orbitals has unpaired electrons in a chlorine radical
Determining Electronic Configurations
- Writing out the electronic configuration tells us how the electrons in an atom or ion are arranged in their shells, subshells and orbitals
- This can be done using the full electron configuration or the shorthand version
- The full electron configuration describes the arrangement of all electrons from the 1s subshell up
- The shorthand electron configuration includes using the symbol of the nearest preceding noble gas to account for however many electrons are in that noble gas
- Ions are formed when atoms lose or gain electrons
- Negative ions are formed by adding electrons to the outer subshell
- Positive ions are formed by removing electrons from the outer subshell
- The transition metals fill the 4s subshell before the 3d subshell but lose electrons from the 4s first and not from the 3d subshell (the 4s subshell is lower in energy)
- The Periodic Table is split up into four main blocks depending on their electronic configuration:
- s block elements
- Have their valence electron(s) in an s orbital
- p block elements
- Have their valence electron(s) in a p orbital
- d block elements
- Have their valence electron(s) in a d orbital
- f block elements
- Have their valence electron(s) in an f orbital
- s block elements
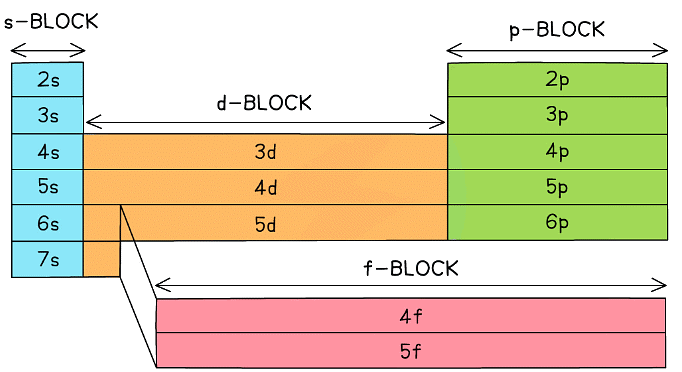 The elements can be divided into four blocks according to their outer shell electron configuration
The elements can be divided into four blocks according to their outer shell electron configuration
Exceptions
- Chromium and copper have the following electron configurations, which are different to what you may expect:
- Cr is [Ar] 3d5 4s1 not [Ar] 3d4 4s2
- Cu is [Ar] 3d10 4s1 not [Ar] 3d9 4s2
- This is because the [Ar] 3d5 4s1 and [Ar] 3d10 4s1 configurations are energetically stable
Example: Electron Configuration
Write down the full and shorthand electron configuration of the following elements:
1. Potassium
2. Calcium
4. Gallium
4. Ca2+
Answer 1: Potassium has 19 electrons so the full electronic configuration is:
1s2 2s2 2p6 3s2 3p6 4s1
The 4s orbital is lower in energy than the 3d subshell and is therefore filled first
The nearest preceding noble gas to potassium is argon which accounts for 18 electrons so the shorthand electron configuration is:
[Ar] 4s1
Answer 2: Calcium has 20 electrons so the full electronic configuration is:
1s2 2s2 2p6 3s2 3p6 4s2
The 4s orbital is lower in energy than the 3d subshell and is therefore filled first
The shorthand version is [Ar] 4s2 since argon is the nearest preceding noble gas to calcium which accounts for 18 electrons
Answer 3: Gallium has 31 electrons so the full electronic configuration is:
[Ar] 3d10 4s2 4p1
Answer 4: What this means is that if you ionise calcium and remove two of its outer electrons, the electronic configuration of the Ca2+ ion is identical to that of argon
Ca2+ is 1s2 2s2 2p6 3s2 3p6
Ar is also 1s2 2s2 2p6 3s2 3p6
Ionisation Energies
- The Ionisation Energy (IE) of an element is the amount of energy required to remove one mole of electrons from one mole of gaseous atoms of an element to form one mole of gaseous ions
- Ionisation energies are measured under standard conditions which are 298 K and 101 kPa
- The units of IE are kilojoules per mole (kJ mol-1)
- The first ionisation energy (IE1) is the energy required to remove one mole of electrons from one mole of atoms of an element to form one mole of 1+ ions
- E.g. the first ionisation energy of gaseous calcium:
Ca(g) → Ca+ (g) + e- IE1 = +590 kJ mol-1
- E.g. the first ionisation energy of gaseous calcium:
Trends in Ionisation Energies
- Ionisation energies show periodicity - a trend across a period of the Periodic Table
- As could be expected from their electron configuration, the group 1 metals have a relatively low ionisation energy, whereas the noble gases have very high ionisation energies
- The size of the first ionisation energy is affected by four factors:
- Size of the nuclear charge
- Distance of outer electrons from the nucleus
- Shielding effect of inner electrons
- Spin-pair repulsion
- First ionisation energy increases across a period and decreases down a group
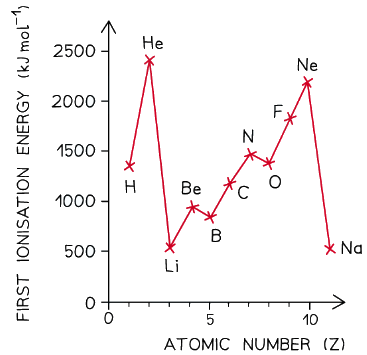 A graph showing the ionisation energies of the elements hydrogen to sodium
A graph showing the ionisation energies of the elements hydrogen to sodium
Ionisation energy across a period
- The ionisation energy across a period generally increases due to the following factors:
- Across a period the nuclear charge increases
- This causes the atomic radius of the atoms to decrease, as the outer shell is pulled closer to the nucleus, so the distance between the nucleus and the outer electrons decreases
- The shielding by inner shell electrons remain reasonably constant as electrons are being added to the same shell
- It becomes harder to remove an electron as you move across a period; more energy is needed
- So, the ionisation energy increases
Dips in the trend
- There is a slight decrease in IE1 between beryllium and boron as the fifth electron in boron is in the 2p subshell, which is further away from the nucleus than the 2s subshell of beryllium
- Beryllium has a first ionisation energy of 900 kJ mol-1 as its electron configuration is 1s2 2s2
- Boron has a first ionisation energy of 800 kJ mol-1 as its electron configuration is 1s2 2s2 2px1
- There is a slight decrease in IE1 between nitrogen and oxygen due to spin-pair repulsion in the 2px orbital of oxygen
- Nitrogen has a first ionisation energy of 1400 kJ mol-1 as its electron configuration is 1s2 2s2 2px1 2py1 2pz1
- Oxygen has a first ionisation energy of 1310 kJ mol-1 as its electron configuration is 1s2 2s2 2px2 2py1 2pz1
- In oxygen, there are 2 electrons in the 2px orbital, so the repulsion between those electrons makes it slightly easier for one of those electrons to be removed
From one period to the next
- There is a large decrease in ionisation energy between the last element in one period, and the first element in the next period
- This is because:
- There is increased distance between the nucleus and the outer electrons as you have added a new shell
- There is increased shielding by inner electrons because of the added shell
- These two factors outweigh the increased nuclear charge
Ionisation energy down a group
The ionisation energy down a group decreases due to the following factors:
- The number of protons in the atom is increased, so the nuclear charge increases
- But, the atomic radius of the atoms increases as you are adding more shells of electrons, making the atoms bigger
- So, the distance between the nucleus and outer electron increases as you descend the group
- The shielding by inner shell electrons increases as there are more shells of electrons
- These factors outweigh the increased nuclear charge, meaning it becomes easier to remove the outer electron as you descend a group
- So, the ionisation energy decreases
Ionisation Energy Trends across a Period & going down a Group Table
Successive ionisation energies of an element
- The successive ionisation energies of an element increase
- This is because once you have removed the outer electron from an atom, you have formed a positive ion
- Removing an electron from a positive ion is more difficult than from a neutral atom
- As more electrons are removed, the attractive forces increase due to decreasing shielding and an increase in the proton to electron ratio
- The increase in ionisation energy, however, is not constant and is dependent on the atom's electronic configuration
- Taking calcium as an example:
Ionisation energies of calcium table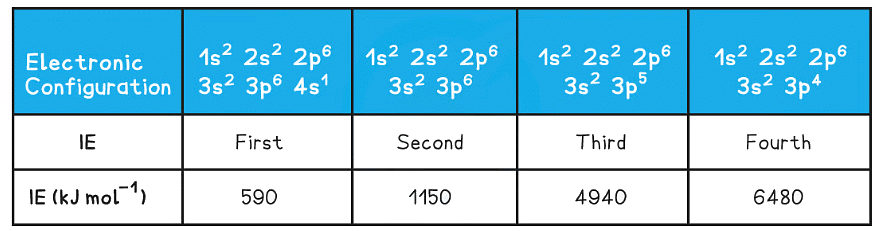
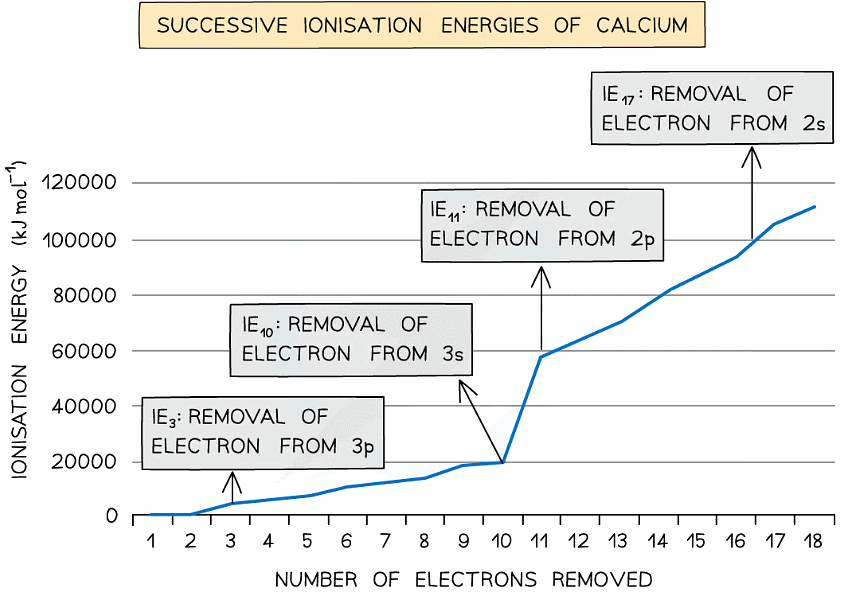
- The first electron removed has a low IE1 as it is easily removed from the atom due to the spin-pair repulsion of the electrons in the 4s orbital
- The second electron is more difficult to remove than the first electron as there is no spin-pair repulsion
- The third electron is much more difficult to remove than the second one corresponding to the fact that the third electron is in a principal quantum shell which is closer to the nucleus (3p)
- Removal of the fourth electron is more difficult as the orbital is no longer full, and there is less spin-pair repulsion
Ionisation Energies: Explained
- Energy is required to remove an outer shell electron as this involves breaking the attractive forces between the electron and the positively charged nucleus
- There are several factors which affect the magnitude of the ionisation energy:
- Nuclear charge
- Positive nuclear charge increases with increasing number of protons
- The greater the positive charge, the greater the attractive forces between the outer electron(s) and the nucleus
- More energy is required to overcome these forces so ionisation energy increases with increasing nuclear charge
- Shielding
- Electrons repel each other and electrons occupying the inner shells repel electrons located in shells further outside the nucleus and prevent them from feeling the full effect of the nuclear charge
- The greater the shielding effect is, the weaker the attractive forces between the positive nucleus and the negatively charged electrons
- Less energy is required to overcome the weakened attractive forces so ionisation energy decreases with increasing shielding effects
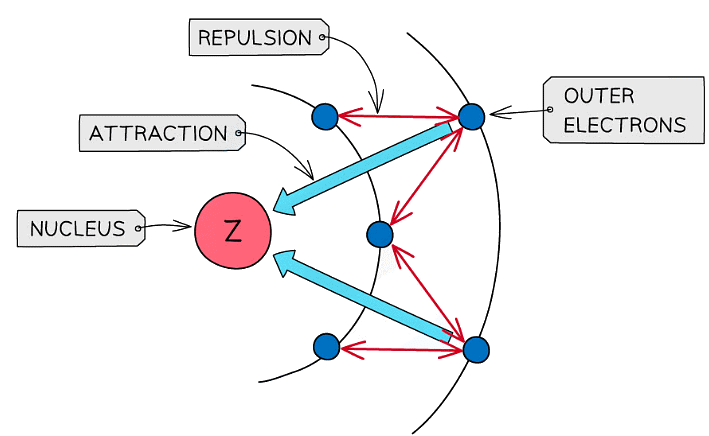 Shielding makes it easier to remove the outermost electrons
Shielding makes it easier to remove the outermost electrons
Atomic/ionic radius
- The larger the radius, the greater the distance between the nucleus and the outer shell electron(s)
- Increasing distance weakens the strength of the attractive forces
- Larger atoms/ions also result in greater shielding due to the presence of more inner electrons
- Less energy is required to remove the outer shell electron(s) so ionisation energy decreases with increasing atomic/ionic radius
Spin-pair repulsion
- Spin pair repulsion occurs when the electron being removed is spin paired with another electron in the same orbital
- The proximity of the like charges of electrons in the orbital results in repulsion
- Less energy is required to remove one of the electrons so ionisation energy decreases when there is spin-pair repulsion
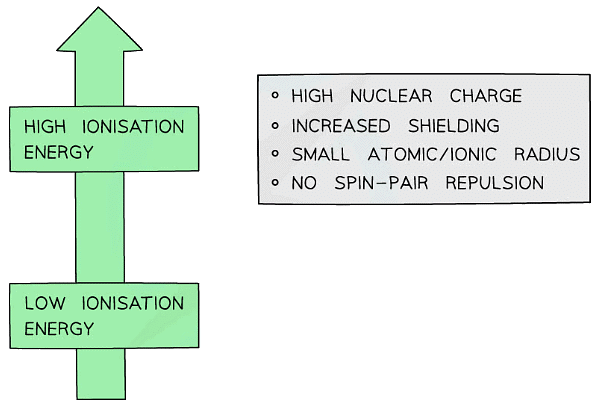 Summary of factors affecting ionisation energies of atoms
Summary of factors affecting ionisation energies of atoms
Ionisation Energies: Electronic Configuration
- Successive ionisation data can be used to:
- Predict or confirm the simple electronic configuration of elements
- Confirm the number of electrons in the outer shell of an element
- Deduce the Group an element belongs to in the Periodic Table
- By analyzing where the large jumps appear and the number of electrons removed when these large jumps occur, the electron configuration of an atom can be determined
- Na, Mg and Al will be used as examples to deduce the electronic configuration and positions of elements in the Periodic Table using their successive ionisation energies
Successive ionisation energies table
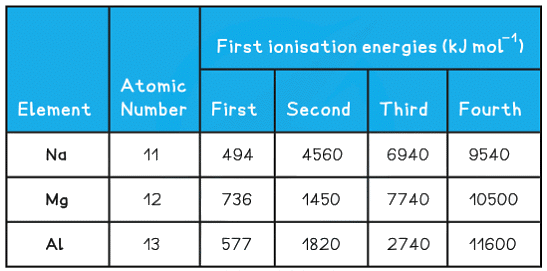
Sodium
- For sodium, there is a huge jump from the first to the second ionisation energy, indicating that it is much easier to remove the first electron than the second
- Therefore, the first electron to be removed must be the last electron in the valence shell thus Na belongs to group I
- The large jump corresponds to moving from the 3s to the full 2p subshell
- Na 1s2 2s2 2p6 3s1
Magnesium
- There is a huge increase from the second to the third ionisation energy, indicating that it is far easier to remove the first two electrons than the third
- Therefore the valence shell must contain only two electrons indicating that magnesium belongs to group II
- The large jump corresponds to moving from the 3s to the full 2p subshell
- Mg 1s2 2s2 2p6 3s2
Aluminium
- There is a huge increase from the third to the fourth ionisation energy, indicating that it is far easier to remove the first three electrons than the fourth
- The 3p electron and 3s electrons are relatively easy to remove compared with the 2p electrons which are located closer to the nucleus and experience greater nuclear charge
- This is due to weakened shielding effects through the loss of three electrons
- The large jump corresponds to moving from the third shell to the second shell
Al 1s2 2s2 2p6 3s2 3p1
|
252 videos|414 docs|264 tests
|





















