Electrode Potentials | Chemistry for Grade 12 PDF Download
Standard electrode potential
- The position of equilibrium and therefore the electrode potential depends on factors such as:
- Temperature
- Pressure of gases
- Concentration of reagents
- So, to be able to compare the electrode potentials of different species, they all have to be measured against a common reference or standard
- Standard conditions also have to be used when comparing electrode potentials
- These standard conditions are:
- Ion concentration of 1.00 mol dm-3
- A temperature of 298 K
- A pressure of 100 kPa
- Standard measurements are made using a high resistance voltmeter so that no current flows and the maximum potential difference is achieved
- The electrode potentials are measured relative to a standard hydrogen electrode
- The standard hydrogen electrode is given a value of 0.00 V, and all other electrode potentials are compared to this standard
- This means that the electrode potentials are always referred to as a standard electrode potential (Eθ)
- The standard electrode potential (Eθ) is the potential difference ( sometimes called voltage) produced when a standard half-cell is connected to a standard hydrogen cell under standard conditions
- For example, the standard electrode potential of bromine suggests that relative to the hydrogen half-cell it is more likely to get reduced, as it has a more positive Eθ value
Br2(l) + 2e– ⇌ 2Br–(aq) Eθ = +1.09 V
2H+(aq) + 2e– ⇌ H2(g) Eθ = 0.00 V - The standard electrode potential of sodium, on the other hand, suggests that relative to the hydrogen half-cell it is less likely to get reduced as it has a more negative Eθ value
Na+ (aq) + e– ⇌ Na(s) Eθ = -2.71 V
2H+ (aq) + 2e– ⇌ H2(g) Eθ = 0.00 V
Electrochemical Cells
- The standard hydrogen electrode is a half-cell used as a reference electrode and consists of:
- Hydrogen gas in equilibrium with H+ ions of concentration 1.00 mol dm-3 (at 100 kPa)
2H+ (aq) + 2e- ⇌ H2 (g) - An inert platinum electrode that is in contact with the hydrogen gas and H+ ions
- Hydrogen gas in equilibrium with H+ ions of concentration 1.00 mol dm-3 (at 100 kPa)
- When the standard hydrogen electrode is connected to another half-cell, the standard electrode potential of that half-cell can be read off a high resistance voltmeter
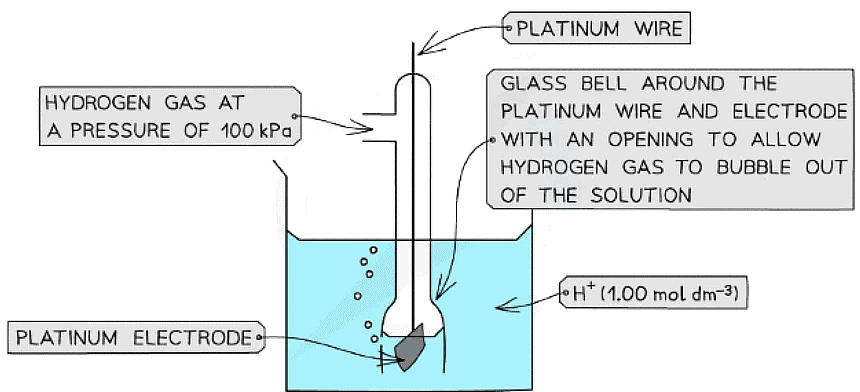
The standard electrode potential of a half-cell can be determined by connecting it to a standard hydrogen electrode
- There are three different types of half-cells that can be connected to a standard hydrogen electrode
- A metal / metal ion half-cell
- A non-metal / non-metal ion half-cell
- An ion / ion half-cell (the ions are in different oxidation states)
Metal / metal-ion half-cell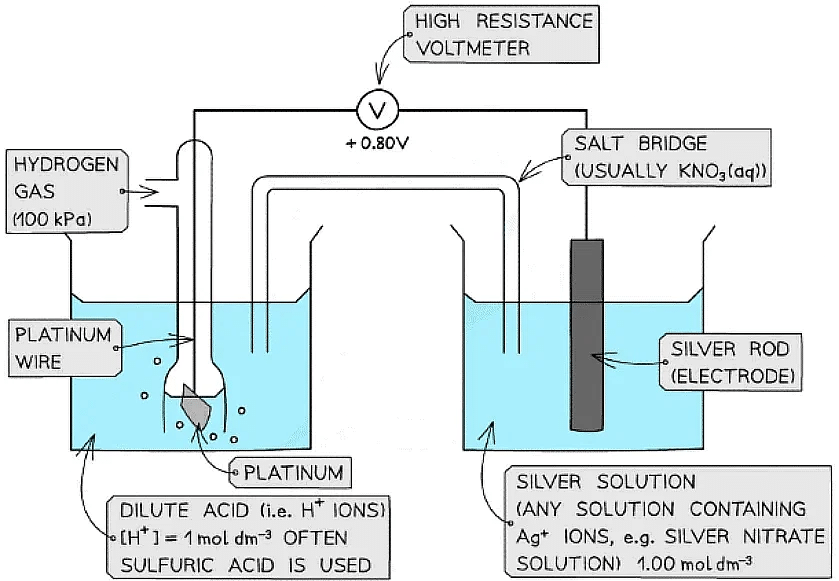
Example of a metal / metal ion half-cell connected to a standard hydrogen electrode
- An example of a metal/metal ion half-cell is the Ag+/ Ag half-cell
- Ag is the metal
- Ag+ is the metal ion
- This half-cell is connected to a standard hydrogen electrode and the two half-equations are:
Ag+ (aq) + e- ⇌ Ag (s) Eꝋ = + 0.80 V
2H+ (aq) + 2e- ⇌ H2 (g) Eꝋ = 0.00 V - Since the Ag+/ Ag half-cell has a more positive Eꝋ value, this is the positive pole and the H+/H2 half-cell is the negative pole
- The standard cell potential (Ecellꝋ) is Ecellꝋ = (+ 0.80) - (0.00) = + 0.80 V
- The Ag+ ions are more likely to get reduced than the H+ ions as it has a greater Eꝋ value
- Reduction occurs at the positive electrode
- Oxidation occurs at the negative electrode
Non-metal / non-metal ion half-cell
- In a non-metal / non-metal ion half-cell, platinum wire or foil is used as an electrode to make electrical contact with the solution
- Like graphite, platinum is inert and does not take part in the reaction
- The redox equilibrium is established on the platinum surface
- An example of a non-metal / non-metal ion is the Br2 / Br- half-cell
- Br2 is the non-metal
- Br- is the non-metal ion
- The half-cell is connected to a standard hydrogen electrode and the two half-equations are:
Br2 (aq) + 2e- ⇌ 2Br- (aq) Eꝋ = +1.09 V
2H+ (aq) + 2e- ⇌ H2 (g) Eꝋ = 0.00 V - The Br2 / Br- half-cell is the positive pole and the H+ / H2 is the negative pole
- The Ecellꝋ is: Ecellꝋ = (+ 1.09) - (0.00) = + 1.09 V
- The Br2 molecules are more likely to get reduced than H+ as they have a greater Eꝋ value
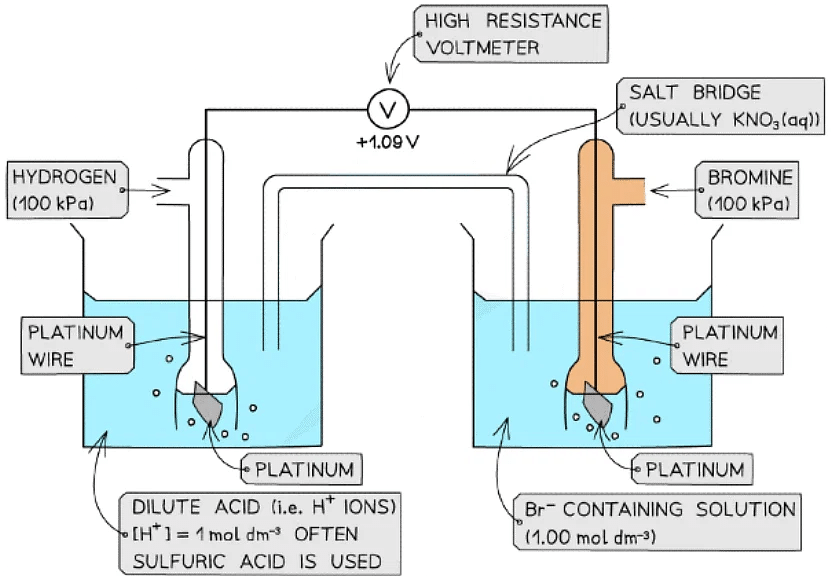
Example of a non-metal / non-metal ion half-cell connected to a standard hydrogen electrode
Ion / Ion half-cell
- A platinum electrode is again used to form a half-cell of ions that are in different oxidation states
- An example of such a half-cell is the MnO4- / Mn2+ half-cell
- MnO4- is an ion containing Mn with oxidation state +7
- The Mn2+ ion contains Mn with oxidation state +2
- This half-cell is connected to a standard hydrogen electrode and the two half-equations are:
MnO4- (aq) + 8H+ (aq) + 5e- ⇌ Mn2+ (aq) + 4H2O (l) Eꝋ = +1.52 V
2H+ (aq) + 2e- ⇌ H2 (g) Eꝋ = 0.00 V - The H+ ions are also present in the half-cell as they are required to convert MnO4- into Mn2+ ions
- The MnO4- / Mn2+ half-cell is the positive pole and the H+ / H2 is the negative pole
- The Ecellꝋ is Ecellꝋ = (+ 1.09) - (0.00) = + 1.09 V
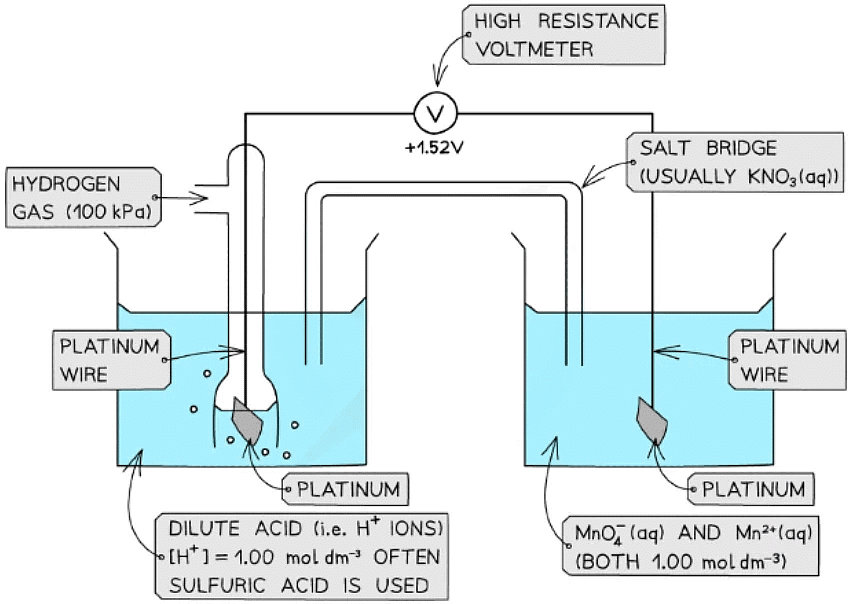
Ions in solution half cell
Conventional Representation of Cells
- Chemists use a type of shorthand convention to represent electrochemical cells
- In this convention:
- A solid vertical (or slanted) line shows a phase boundary, that is an interface between a solid and a solution
- A double vertical line (sometimes shown as dashed vertical lines) represents a salt bridge
(i) A salt bridge has mobile ions that complete the circuit
(ii) Potassium chloride and potassium nitrate are commonly used to make the salt bridge as chlorides and nitrates are usually soluble
(iii) This should ensure that no precipitates form which can affect the equilibrium position of the half cells - The substance with the highest oxidation state in each half cell is drawn next to the salt bridge
- The cell potential difference is shown with the polarity of the right hand electrode
- The cell convention for the zinc and copper cell would be
Zn (s)∣Zn2+ (aq) ∥Cu2+ (aq)∣Cu (s) Ecell = +1.10 V - This tells us the copper half cell is more positive than the zinc half cell, so that electrons would flow from the zinc to the copper
- The same cell can be written as:
Cu (s)∣Cu2+ (aq) ∥Zn2+ (aq)∣Zn (s) Ecell = -1.10 V - The polarity of the right hand half cell is negative, so we can still tell that electrons flow from the zinc to the copper half cell
Example: Writing a cell diagram
If you connect an aluminium electrode to a zinc electrode, the voltmeter reads 0.94V and the aluminium is the negative. Write the conventional cell diagram to the reaction.
Al (s)∣Al3+ (aq) ∥ Zn2+ (aq)∣Zn (s) Ecell = +0.94 V
It is also acceptable to include phase boundaries on the outside of cells as well:
∣ Al (s)∣Al3+ (aq) ∥ Zn2+ (aq)∣Zn (s) ∣ Ecell = +0.94 V
Calculating Standard Cell Potential
- Once the Eꝋ of a half-cell is known, the potential difference or voltage or emf of an electrochemical cell made up of any two half-cells can be calculated
- These could be any half-cells and neither have to be a standard hydrogen electrode
- The standard cell potential (Ecellꝋ) can be calculated by subtracting the less positive Eꝋ from the more positive Eꝋ value
- The half-cell with the more positive Eꝋ value will be the positive pole
- By convention this is shown on the right hand side in a conventional cell diagram, so is termed Erightꝋ
- The half-cell with the less positive Eꝋ value will be the negative pole
- By convention this is shown on the left hand side in a conventional cell diagram, so is termed Eleftꝋ
Ecellꝋ = Erightꝋ - Eleftꝋ
- Since oxidation is always on the left and reduction on the right, you can also use this version
Ecellꝋ = Ereductionꝋ - Eoxidation
Example: Calculating the standard cell potential
Calculate the standard cell potential for the electrochemical cell below and explain why the Cu2+ / Cu half-cell is the positive pole. The half-equations are as follows:
Cu2+(aq) + 2e- ⇌ Cu(s) Eꝋ = +0.34 V
Zn2+(aq) + 2e- ⇌ Zn(s) Eꝋ = −0.76 V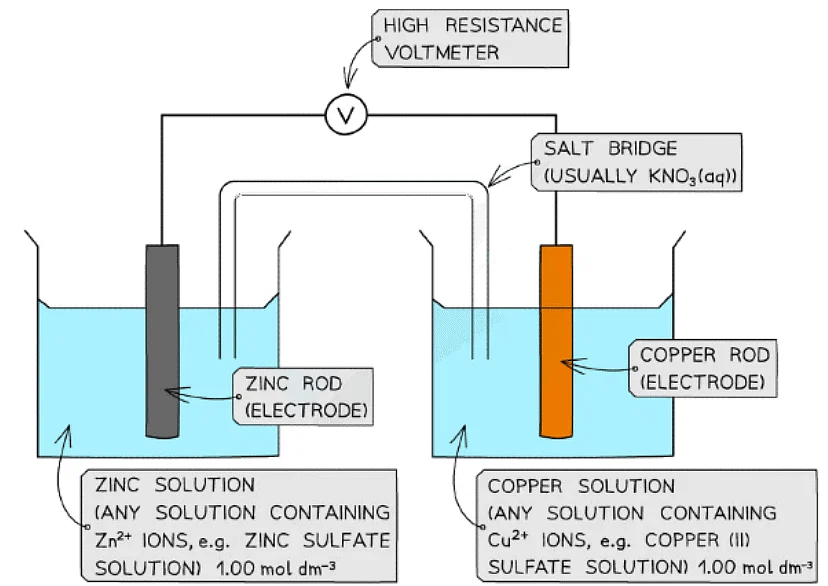
Step 1: Calculate the standard cell potential. The copper is more positive so must be the right hand side.
Ecellꝋ = Erightꝋ - Eleftꝋ
Ecellꝋ = (+0.34) - (-0.76)
= +1.10 V
The voltmeter will therefore give a value of +1.10 VStep 2: Determine the positive and negative poles
The Cu2+ / Cu half-cell is the positive pole as its Eꝋ is more positive than the Eꝋ value of the Zn2+ / Zn half-cell
 |
Download the notes
Electrode Potentials
|
Download as PDF |
Feasibility & Standard Cell Potential
Feasibility
- The Eꝋ values of a species indicate how easily they can get oxidised or reduced
- The more positive the value, the easier it is to reduce the species on the left of the half-equation
- The reaction will tend to proceed in the forward direction
- The less positive the value, the easier it is to oxidise the species on the right of the half-equation
- The reaction will tend to proceed in the backward direction
- A reaction is feasible (likely to occur) when the Ecellꝋ is positive
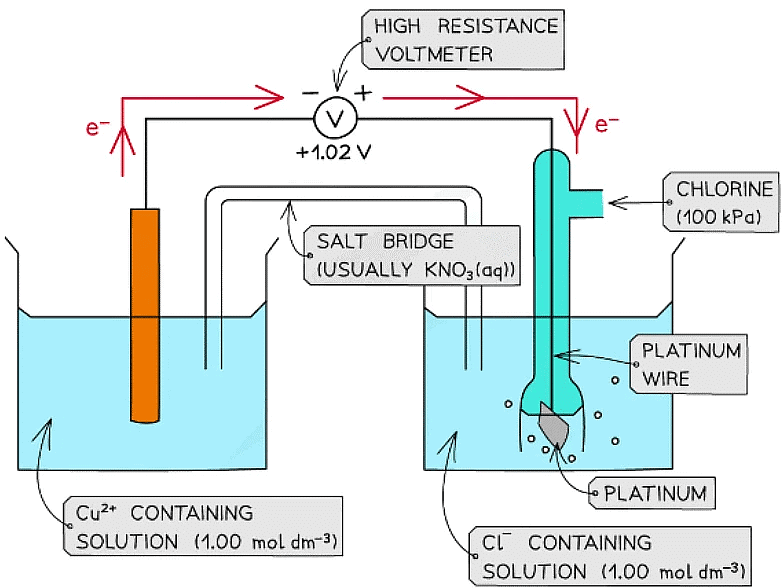
- For example, two half-cells in the following electrochemical cell are:
Cl2 (g) + 2e- ⇌ 2Cl- (aq) Eꝋ = +1.36 V
Cu2+ (aq) + 2e- ⇌ Cu (s) Eꝋ = +0.34 V - Cl2 molecules are reduced as they have a more positive Eꝋ value
- The chemical reaction that occurs in this half cell is:
Cl2 (g) + 2e- → 2Cl- (aq) - Cu2+ ions are oxidised as they have a less positive Eꝋ value
- The chemical reaction that occurs in this half cell is
Cu (s) → Cu2+ (aq) + 2e- - The overall equation of the electrochemical cell is (after cancelling out the electrons):
Cu (s) + Cl2 (g) → 2Cl- (aq) + Cu2+ (aq)
OR
Cu (s) + Cl2 (g) → CuCl2 (s) - The forward reaction is feasible (spontaneous) as it has a positive Eꝋ value of +1.02 V ((+1.36) - (+0.34))
- The backward reaction is not feasible (not spontaneous) as it has a negative Eꝋ value of -1.02 ((+0.34) - (+1.36))
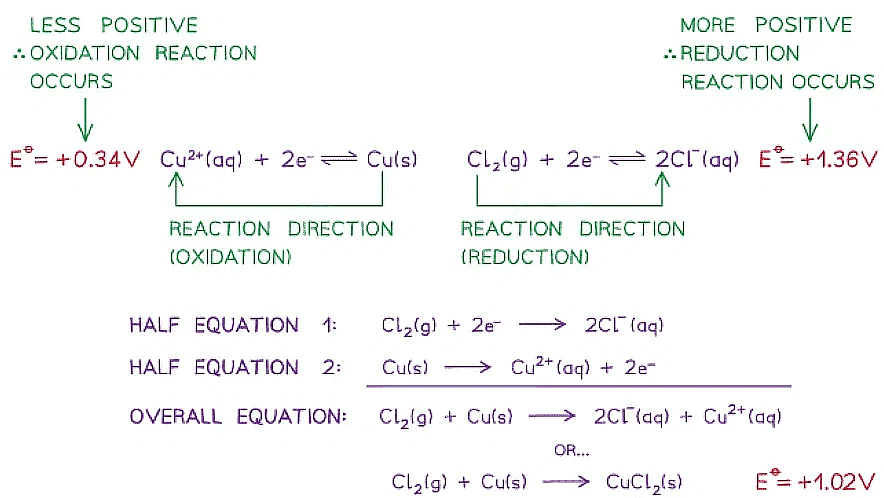
A reaction is feasible when the standard cell potential Eꝋ is positive
Limitations of Eθ to predict reactions
- The thermodynamic feasibility of a reaction can be deduced from the electrode potential, however, it gives no information about the rate of reaction
- As standard electrode potentials are measured using solutions, we have to consider the le Châtelier's effect on concentration using non-standard conditions
- For example, the redox equilibrium equation and standard electrode potential for the V3+ | V2+ system are:
V3+ (aq) + e- ⇌ V2+ (aq) Eθ = +0.26 V - If the concentration of V3+ (aq) is greater than 1.0 mol dm-3, then the equilibrium will shift to the right
- This will remove electrons from the system, therefore, making the electrode potential less negative
- If the concentration of V2+ (aq) is greater than 1.0 mol dm-3, then the equilibrium will shift to the left
- This will add electrons to the system, therefore, making the electrode potential more negative
- Any change to the concentration will cause a change to the electrode potential and, therefore, to the overall cell potential
- This is true of any change to the conditions that results in non-standard conditions
- Another, more basic limitation is the fact that many redox reactions are not aqueous
- For example, the redox equilibrium equation and standard electrode potential for the V3+ | V2+ system are:
|
207 videos|373 docs|227 tests
|




















