Atoms, Elements and Compounds | Chemistry for Grade 10 PDF Download
Chemical symbols
- All substances are made from tiny particles called atoms. An atom is the smallest part of an element that can exist.
- Atoms of each element are represented by their own chemical symbol. A chemical symbol:
- consists of one or two letters
- always starts with a capital letter, with any other letter in lower case
- For example the symbol O represents an atom of oxygen, and Na represents an atom of sodium. You must write the chemical symbol of sodium as Na, not as NA, na or nA.
- There are over 100 different elements. The names and symbols of the elements are shown in the periodic table. Elements are arranged into groups with similar properties. Groups are numbered from 1 to 7, then 0.
- In the periodic table, metals are on the left of the stepped line, and non-metals are on the right.
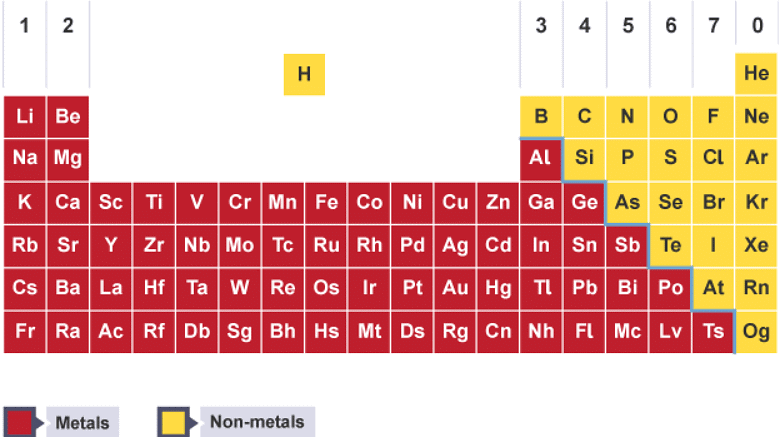
Each box in the periodic table shows the chemical symbol for an element
Chemical formulae of elements
A chemical formula is used to represent an element or compound in balanced chemical equations.
The formula for most elements is just its chemical symbol. For example:
- helium, He
- lithium, Li
- beryllium, Be
- boron, B
- carbon, C
- neon, Ne
- sodium, Na
- magnesium, Mg
It's important to use the names and chemical symbols for the first 20 elements in the periodic table, as well as the elements in groups 1 and 7.
Some non-metal elements exist as molecules that are made up of two atoms joined together. The formulae of these elements are the element's symbol followed by a subscripted '2'. For example:
- iodine, I2
- bromine, Br2
- chlorine, Cl2
- fluorine, F2
- oxygen, O2
- nitrogen, N2
- hydrogen, H2
Chemical formulae of compounds
- A compound is a substance that contains two or more elements that are chemically combined. The elements in a compound are present in fixed proportions. For example, carbon dioxide always has 12 g of carbon for every 32 g of oxygen.
- A chemical formula can be used to represent a compound. The formula shows:
- the symbols for each element in the compound
- the number of atoms of each element in a unit of the compound
- For example, magnesium oxide is made up of two elements, magnesium and oxygen. Its formula is MgO. This shows that it has one atom of magnesium for every one atom of oxygen.
- Here are some more examples of compounds and their formulae. The subscript number in a formula shows if there is more than one atom of an element.
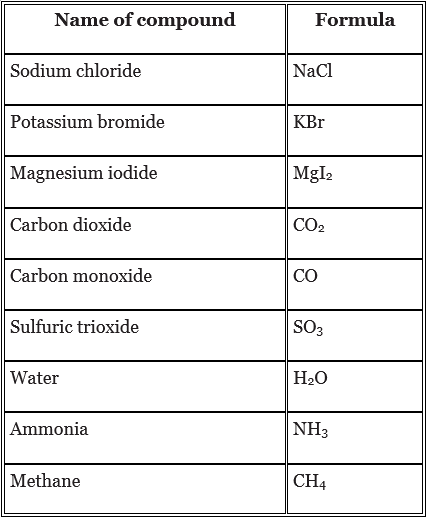
- Many compounds exist naturally. They can also be formed from their elements in chemical reactions. In a chemical reaction, one or more new substances are formed. Most chemical reactions involve energy changes.
- It is not easy to split up a compound into its elements - the only way to do this is in chemical reactions.
In compounds made up of non-metal elements only, the second word of the compound's name starts with mon-, di-, or tri-, eg carbon dioxide. This shows the number of atoms of this element for every one atom of the first element in the name. So for carbon dioxide there are two oxygen atoms for every carbon atom.
Chemical formulae of ions
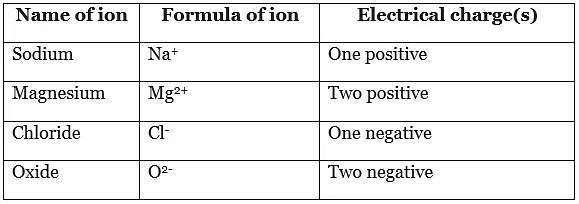
- An ion is a charged particle formed when an atom, or a group of atoms, loses or gains electrons. The number and sign of its electrical charges are shown in superscript text.
- Names and formulae of some common ions:
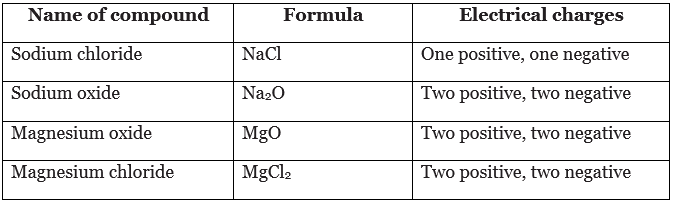
Simple formulae
- The formula of an ionic compound can be predicted using the formulae of its ions. The numbers of ions in a formula must give an equal number of positive and negative charges.
Word equations
- A word equation represents a chemical reaction using the names of the substances involved. Word equations do not show any chemical symbols or formulae.
Reactants and products
- Reactants are substances that react together in a chemical reaction. In a chemical reaction, the atoms or ions in reactants separate from one another. They join back together in a different way to form products.
Word equations always take this form:
- reactants → products
- A + sign separates two or more reactants, or two or more products.
Example word equations
- Potassium hydroxide reacts with sulfuric acid. Potassium sulfate and water are formed in the reaction. This means that:
- the reactants are potassium hydroxide and sulfuric acid
- the products are potassium sulfate and water
- the word equation is: potassium hydroxide + sulfuric acid → potassium sulfate + water
Chemical equations contain an arrow and not an equals sign. The arrow means 'reacts to make'.
There can be different numbers of reactants and products. For example:
- sodium + chlorine → sodium chloride
- calcium carbonate → calcium oxide + carbon dioxide
Balanced chemical equations
- A balanced chemical equation represents a chemical reaction using the formulae of the reactants and products. It shows the number of units of each substance involved.
State symbols
- Balanced chemical equations sometimes include state symbols in brackets after each formula. They show the physical state of that substance.
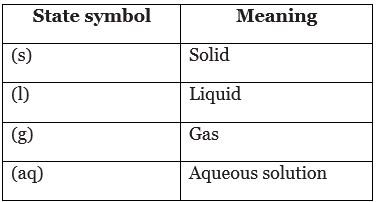
- An aqueous solution forms when a substance dissolves in water.
State symbols are useful because they show what a substance is like. For example:
- H2O(l) is liquid water but H2O(g) is steam and H2O(s) is ice
- HCl(g) is hydrogen chloride gas but HCl(aq) is hydrochloric acid
Balancing an equation
- The law of conservation of mass states that no atoms are lost or made during a chemical reaction, so the total mass of the products is equal to the total mass of the reactants.
- This means that chemical reactions can be represented by symbol equations. A balanced symbol equation has the same number of atoms of each element on both sides of the arrow.
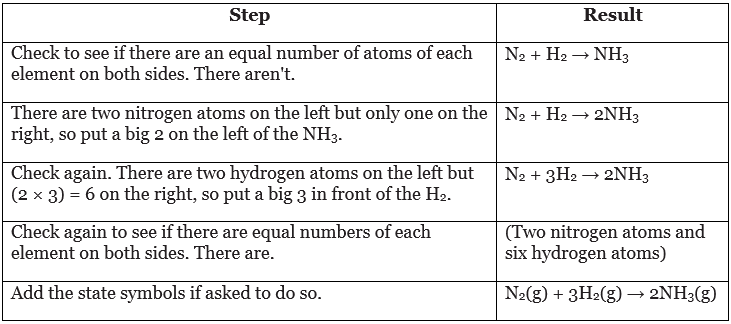
- To balance an equation, add numbers to the left of one or more formulae. Here is one way to work out how to do this for the reaction between nitrogen and hydrogen.
|
82 videos|200 docs|26 tests
|





















