Groups in the periodic table | Chemistry for Grade 10 PDF Download
Group 0 - physical properties
- Group 0 contains non-metal elements placed in the vertical column on the far right of the periodic table. The elements in group 0 are called the noble gases. They exist as single atoms.
Group 0 is on the far right-hand side of the periodic table
- The noble gases show trends in their physical properties.
Boiling points
- The noble gases all have low boiling points:
- helium, at the top of group 0, has the lowest boiling point of any element
- the attractive forces between the atoms become stronger
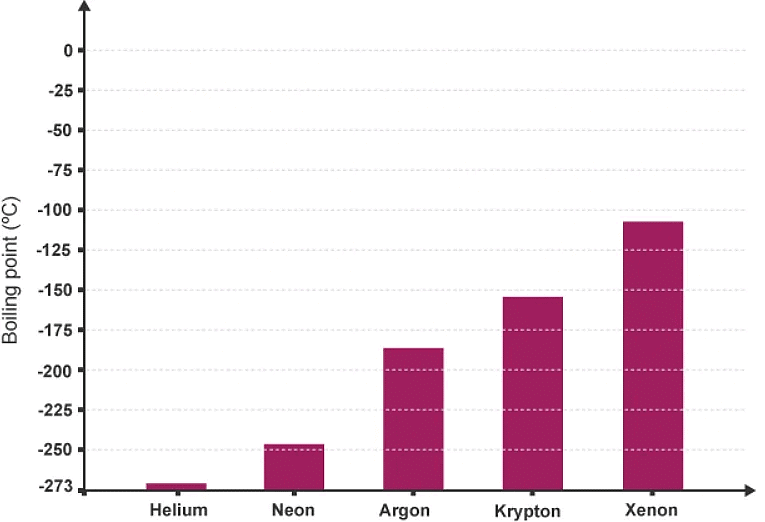
This is because, going down group 0:
- the atoms become larger
- the intermolecular forces between the atoms become stronger
- more energy is needed to overcome these forces
Example: Radon is situated below xenon in group 0. Predict the likely boiling point of radon.
The actual boiling point of radon is -61.7˚C. An estimate would lie midway between -100˚C and -50˚C based on the shape of the graph.
Group 0 - chemical properties
- Compared to other elements, the noble gases are inert - they are extremely unreactive and do not take part in chemical reactions.
Explaining the inertness of noble gases
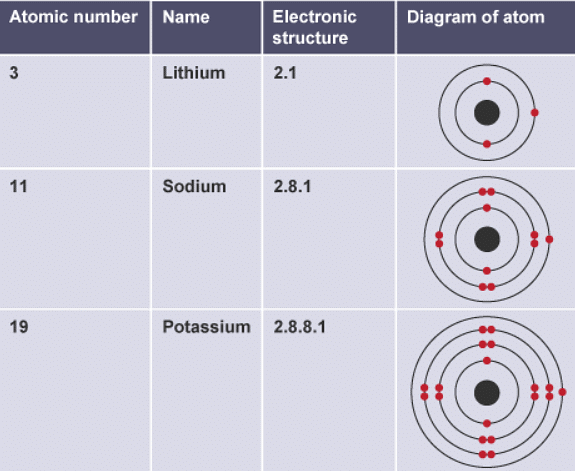
- The table shows the electronic structures of some noble gases. All the noble gases have complete outer shells:
- helium has only two electrons
- the other elements have eight electrons in the outer shell
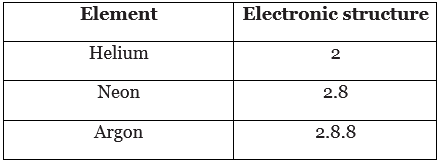
- When elements react, their atoms complete their outer shells by losing, gaining, or sharing electrons. Atoms of group 0 elements already have stable arrangements of electrons. This means that they have no tendency to lose, gain, or share electrons. This is why the noble gases are unreactive. It also explains why atoms of group 0 elements do not share electrons to form molecules.
Group 1 - physical properties
- Group 1 contains elements placed in a vertical column on the far left of the periodic table. The elements in group 1 are called the alkali metals.
Group 1 is on the left-hand side of the periodic table
The alkali metals share similar physical properties. For example, they:
- are soft (they can be cut with a knife)
- have relatively low melting points
- have low densities
Example: The table shows the melting points of five alkali metals. Use this information to describe how melting point changes in group 1.
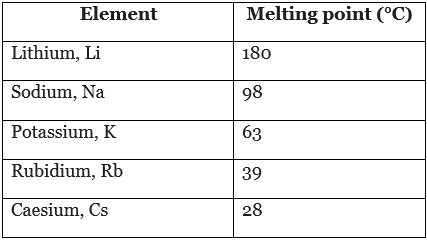
Going down group 1, the melting point decreases.
Group 1 - chemical reactions with water
- Atoms of group 1 elements all have one electron in their outer shell. This means that the alkali metals all have similar chemical properties.
- When a group 1 element takes part in a reaction, its atoms each lose one electron. This forms positively charged ions. The ions have a stable arrangement of electrons, with a complete outer shell.
Reactions with water
- The alkali metals react with water to produce a metal hydroxide and hydrogen. For example, sodium reacts with water:
- sodium + water → sodium hydroxide + hydrogen
- 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
- Sodium hydroxide is an alkali. It is a base that dissolves in water to form an alkaline solution. This solution:
- has a pH greater than 7
- turns universal indicator solution blue or purple
Example: Write the word equation and balanced symbol equation for the reaction of potassium with water.
potassium + water → potassium hydroxide + hydrogen
2K(s) + 2H2O(l) → 2KOH(aq) + H2(g)
Reactions compared
- The table shows observations when lithium, sodium and potassium are added to water. Notice that the reactivity of these metals increases going down the group. This pattern is seen with all reactions of group 1 elements.
- For example, the reaction of caesium with chlorine is more vigorous than the reaction of potassium with chlorine.

The reaction of potassium with water gives a lilac flame

Group 1 - chemical reactions with oxygen and chlorine
Reactions with oxygen
- The group 1 elements react with oxygen from the air to make metal oxides.
- At room temperature, oxygen reacts with the surface of the metal. This forms a white oxide, which covers the surface. The metal below the surface does not react.
- The alkali metals burn vigorously when heated and placed in a gas jar of oxygen. The oxide forms as white smoke.
- For example:
sodium + oxygen → sodium oxide
4Na(s) + O2(g) → 2Na2O(s) - The reactivity of the group 1 elements increases down the group, so their reactions with oxygen get more vigorous.
Example: Predict which becomes white more quickly on exposure to air - a piece of rubidium, or a piece of lithium. Explain your answer.
The rubidium becomes white more quickly. This is because rubidium is below lithium in group 1, so rubidium is more reactive.
Reactions with chlorine
- The group 1 elements react vigorously with chlorine. The products of the reactions are chlorides. At room temperature the chlorides are white solids. They dissolve in water to form colourless solutions. For example:
sodium + chlorine → sodium chloride
2Na(s) + Cl2(g) → 2NaCl(s) - The reactions with chlorine get more vigorous going down the group.
Explaining the trend in reactivity
- When a group 1 element takes part in a reaction, each of its atoms loses its outer electron to form a positively charged ion. The more easily the ions form, the more reactive the metal.
- Going down group 1:
- the atoms become larger
- the outer electron becomes further from the nucleus
- the force of attraction between the nucleus and the outer electron decreases
- the outer electron is lost more easily
Group 7 - physical properties
- Group 7 contains non-metal elements placed in a vertical column on the right of the periodic table. The elements in group 7 are called the halogens.
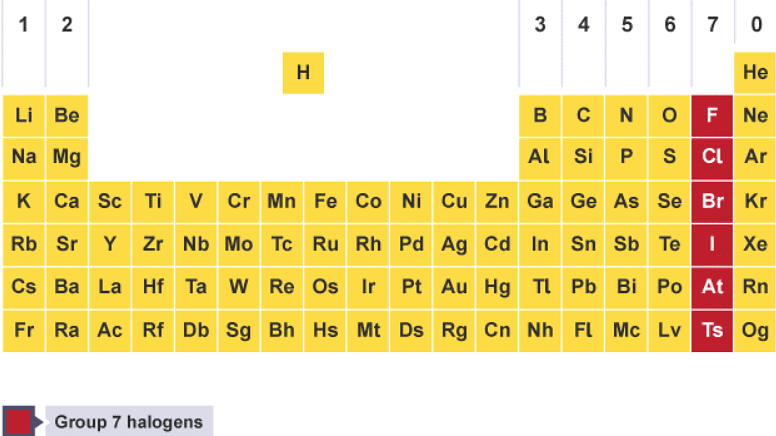
Group 7 is on the right-hand side of the periodic table, next to group 0
- The halogens show trends in their physical and chemical properties.
Physical properties
- The halogens exist as simple molecules. Each molecule is made up of a pair of halogen atoms joined by a single covalent bond. In all groups of the periodic table, the further down the group an element is, the higher its relative molecular mass.
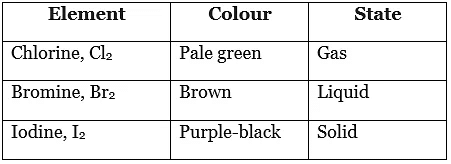
- The table shows the colour and physical states of chlorine, bromine and iodine at room temperature.
In group 7, the further down the group an element is, the higher its melting point and boiling point. This is because, going down group 7:
- the molecules become larger
- the intermolecular forces become stronger
- more energy is needed to overcome these forces
Example: The graph shows the melting and boiling points of the first four group 7 elements. Astatine is placed below iodine in group 7. Predict the melting and boiling points of astatine, and its state at room temperature.
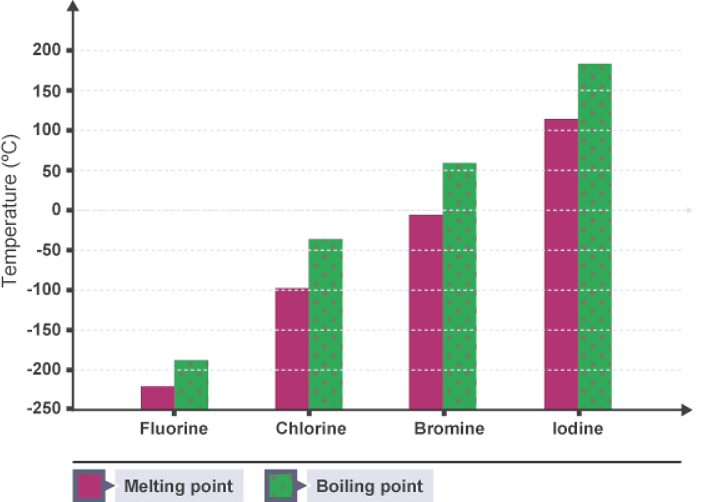
Astatine should have a melting point of about 300°C and a boiling point of about 340°C. This means that it will be solid at room temperature.
Group 7 - chemical properties
- Atoms of group 7 elements all have seven electrons in their outer shell. This means that the halogens all have similar chemical reactions.
- When a group 7 element takes part in a reaction, its atoms each gain one electron. These atoms form negatively charged ions. The ions have a stable arrangement of electrons, with a complete outer shell.
Reactions with metals
- The halogens react with metals to produce salts. The salts are made up of ions, which are held together by ionic bonds. For example, chlorine reacts with sodium:
sodium + chlorine → sodium chloride
2Na(s) + Cl2(g) → 2NaCl(s) - Sodium and chlorine react vigorously when heated, giving an orange flame and clouds of white sodium chloride.
- In group 7, the reactivity of the elements decreases down the group. The table describes what happens when halogens react with iron wool.
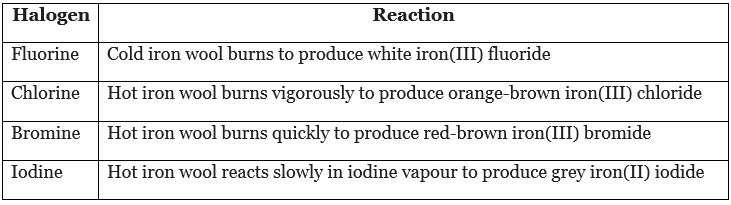
Example: Write a balanced equation for the reaction of iron with chlorine to produce solid iron(III) chloride, FeCl3. Include state symbols.
2Fe(s) + 3Cl2(g) → 2FeCl3(s)
Reactions with non-metals
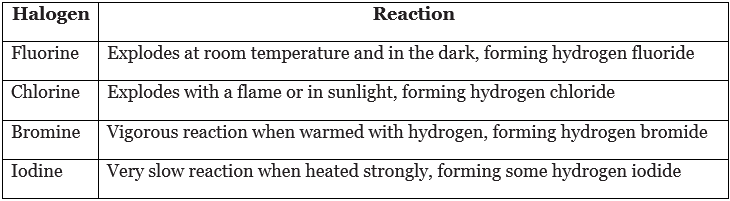
- The halogens react with non-metals such as hydrogen. When a halogen reacts with hydrogen, the product is a compound called a hydrogen halide. For example, chlorine reacts with hydrogen:
hydrogen + chlorine → hydrogen chloride
H2(g) + Cl2(g) → 2HCl(g) - The hydrogen halides are gases at room temperature. They dissolve in water to produce acidic solutions. Hydrogen chloride dissolves in water to produce hydrochloric acid, HCl(aq).
- The table describes what happens when halogens react with hydrogen. It shows that the reactivity of the elements decreases down the group.
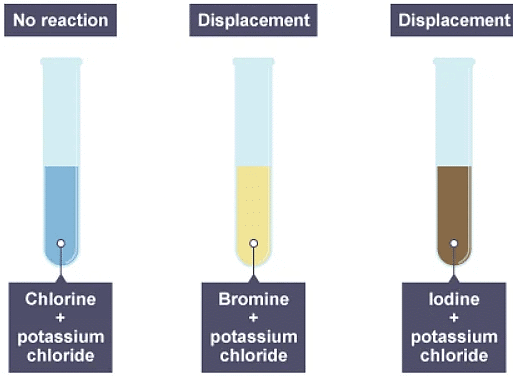
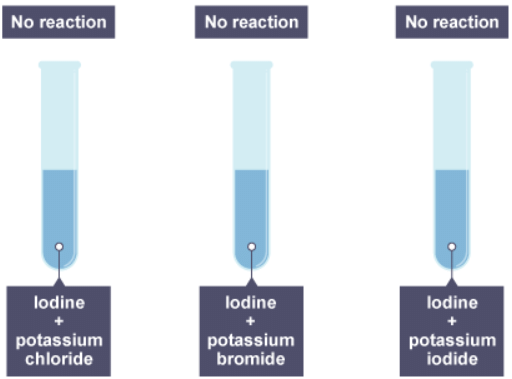
Group 7 - displacement reactions
- A more reactive halogen can displace a less reactive halogen from solutions of its salts. For example, chlorine is more reactive than iodine. A solution of chlorine can displace iodine from potassium iodide solution:
chlorine + potassium iodide → potassium chloride + iodine
Cl2(aq) + 2KI(aq) → 2KCl(aq) + I2(aq) - The reaction mixture turns darker as iodine solution forms.
Adding chlorine, bromine and iodine to halogen salts
1. Chlorine water is added to three solutions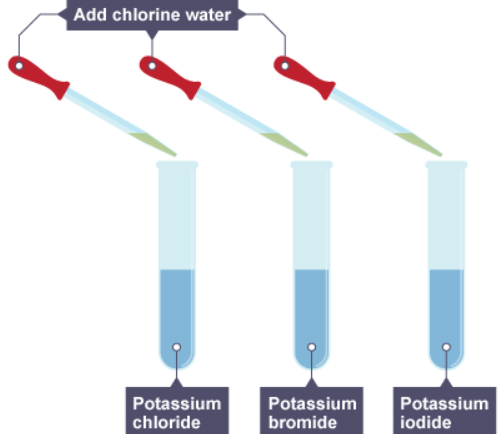
2. The result of adding chlorine to the three solutions
3. Bromine water is added to three solutions
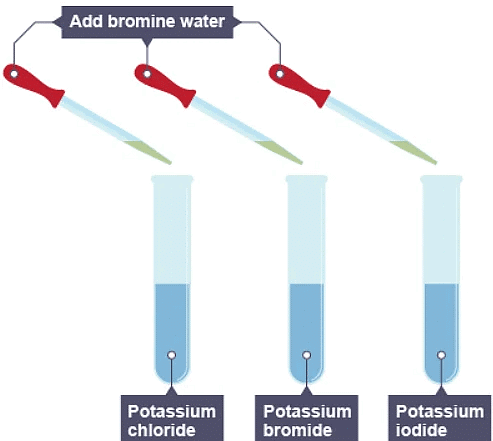
4. The result of adding bromine to the three solutions
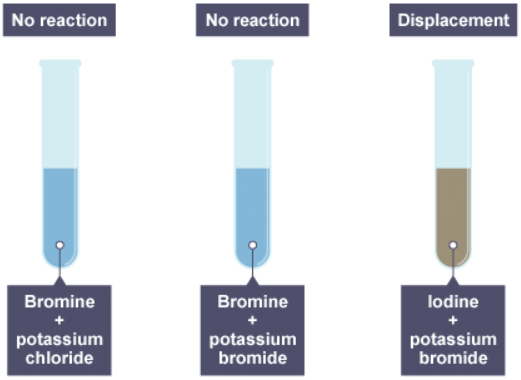
5. Iodine water is added to three solutions
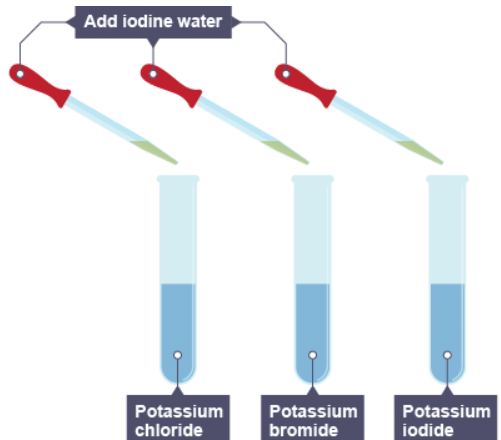
6. The result of adding iodine to the three solutions
Example: Write a balanced equation for the displacement reaction of bromine solution with sodium iodide solution.
Br2(aq) + 2NaI(aq) → 2NaBr(aq) + I2(aq)
|
82 videos|200 docs|26 tests
|















