Protein Structure | Biology and Biochemistry for MCAT PDF Download
Proteins are made up of amino acids which undergo various stages of folding to form their shape and structure. Depending on the structure of the protein they will have different functions within the body, including structural, regulatory, contractile and protective roles.
Amino Acids
Amino acids are the basic building blocks of proteins. Their structure consists of three main groups as seen in figure 1, namely the amino group or N terminus, the carboxyl group or C terminus, and the R group which contains the functional component of the amino acid. The R group gives the amino acid specific features according to its polarity and charge, which then affect the chemical and biological properties of the protein.
There are a total of 21 amino acid types based on their different R groups. 12 of these can be synthesised in the body, while the other 9 must be consumed in the diet and are termed essential amino acids.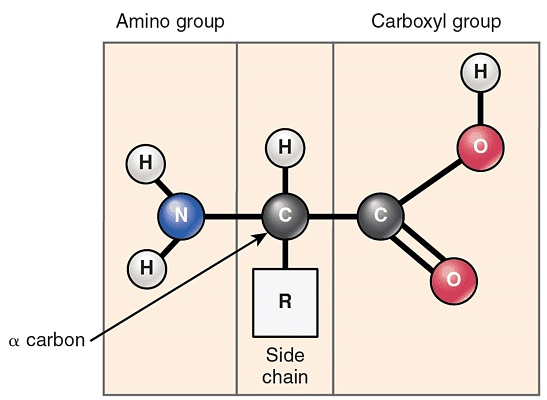 Diagram showing the basic structure of an amino acid.
Diagram showing the basic structure of an amino acid.
Protein structure
Protein structure can be divided into four main categories depending on the level of complexity.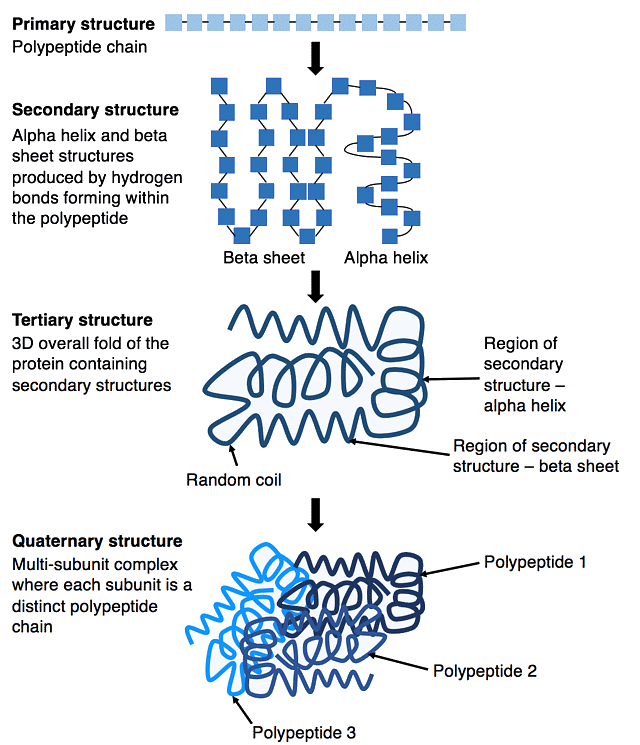
Primary Protein Structure
Primary protein structure is when amino acids bound are together via covalent peptide bonds to form a polypeptide chain. These bonds form between the N terminal and C terminal of amino acids and are highly resistant to heat or chemicals. Any mutation in this amino acid sequence can affect protein folding, leading to problems with the protein’s function.
Secondary Protein Structure
Secondary protein structure is the repetitive folding of polypeptide chains by hydrogen bonds between the hydroxyl (OH) group and the hydrogen molecule of the adjacent amino acid, leading to the unique shape of the protein. The most common examples are the alpha-helix and beta-pleated sheets.
- Alpha-helix – a coil formed by hydrogen bonds between the carbonyl group and the amino group of different amino acids. The strong bonds and stability of this structure give it a strong tensile strength, which allows it to form the shape seen in DNA.
- Beta-pleated sheet – formed by hydrogen bonds between the carboxyl group of one amino acid on one sheet and the hydrogen molecule of an amino acid on another sheet. The sheets can be in parallel or antiparallel.
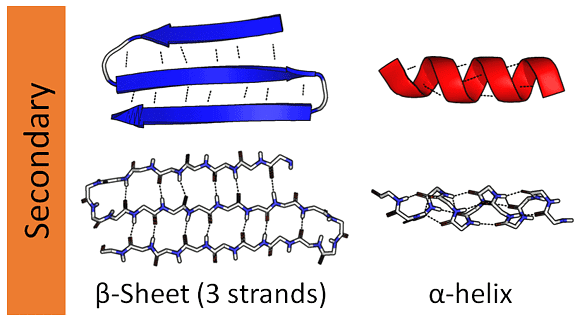
Tertiary Protein Structure
Tertiary protein structure is the folding of the polypeptide chain into a unique 3D structure. This tends to be globular in shape and contains a binding site for the protein action. Folding of the polypeptide chain occurs via an interaction between the R groups of amino acids.
The tertiary structure can therefore be deranged if there is disruption to the bonds between R groups, causing the structure to lose its shape and resulting in a loss of function. This is known as protein denaturation.
The type of bonds involved in the formation of the tertiary protein structure include hydrogen bonds, electrostatic or ionic bonds, covalent bonds or hydrophobic bonds.
- Hydrostatic bonds – form between the hydroxyl (OH) group and an adjacent hydrogen molecule, providing a strong bond between polar R groups.
- Electrostatic bonds – form between positive and negative charge. They can be disrupted by the presence of other charged molecules near them.
- Covalent disulphide bonds – form between sulphide groups within the R group of amino acids. They usually occur between two cysteine amino acids, which contain sulphur within their R groups.
- Hydrophobic bonds – form between non-polar groups and commonly involve the benzene group.
Quaternary Protein Structure
Quaternary protein structure is when multiple polypeptide chains link together to form a functioning unit. It is formed via bonds between the R groups of different amino acids within the polypeptide chains, which help to give the protein its shape.
At this point, the protein is fully functional and able to perform its specific role(s) within the body. Examples of fully functional proteins include hormones like insulin, haemoglobin, enzymes and intracellular signalling structures.
Clinical Relevance - Sickle Cell Disease
Normal haemoglobin has a globular shape due to the complex folding patterns of its polypeptide chains, which allows it to perform its function in oxygen transport.
However, in sickle cell disease, a genetic mutation leads to a change in an amino acid from glutamic acid to valine. This changes the shape of the haemoglobin subunit and hence leads to changes in its folding. This underpins the pathophysiology of sickle cell disease.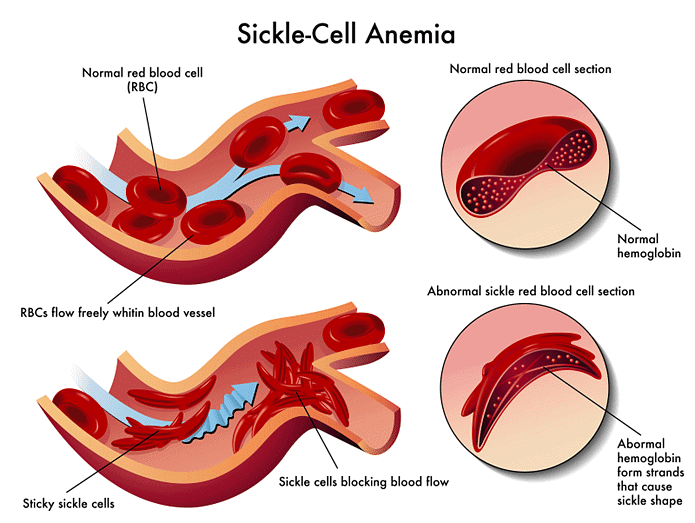
|
129 videos|60 docs|24 tests
|
















