Phosphorus | Biology and Biochemistry for MCAT PDF Download
Compounds of Phosphorus
Compounds of Phosphorus: Phosphorus is a chemical element having atomic number 15 and is represented by its chemical symbol ‘P’. It is a highly reactive element and is present in different allotropic forms in nature as Red Phosphorus, White Phosphorus, and Black Phosphorus. In minerals, Phosphorus is found in the form of Phosphates. Phosphorus forms several compounds with other elements like oxygen, nitrogen, hydrogen, sulphur, and halogens.
Some of the compounds of Phosphorus include Phosphates, Phosphorus pentoxide, Phosphorus trichloride, Phosphorus pentafluoride, phosphides, phosphines, Phosphorus oxoacids, and many more. All these compounds have different structures, properties and uses. Phosphorus forms inorganic as well as organic compounds that are very useful in nature. But some Phosphorus compounds are also toxic. In this article, we will learn about different compounds of Phosphorus extensively.
What is Phosphorus?
Phosphorus was discovered by a German merchant in1669. It is a chemical element that is represented by its chemical symbol 'P' and has atomic number 15. Based on the position of Phosphorus in the modern periodic table, it is a p-block element present in group 15 and third period. The electronic configuration of Phosphorus is [Ne]3s23p3
Red Phosphorus and white Phosphorus are the two forms in which they exist. Phosphorus is highly reactive in nature and hence, always found in a combined state rather than in a free state. Phosphorus is usually present as ‘phosphate’ in minerals.
Allotropes of Phosphorus
1. White Phosphorus – It is a translucent, white, and waxy solid. It is the most reactive, the least dense, and the most toxic allotrope of Phosphorus. When white Phosphorus is exposed to light and heat, it gradually changes to red Phosphorus.
2. Red Phosphorus – Red Phosphorus is an allotrope of Phosphorus having a polymeric structure. One additional bond is formed with the neighbouring tetrahedron atom of P4 the molecule comes to form a chain-like structure. Red Phosphorus can also be formed by heating white Phosphorus at 250oC or by exposing white Phosphorus to the sunlight.
3. Black Phosphorus – Black Phosphorus is formed when red Phosphorus is heated in a sealed tube at 803K. α-black and β-black are the two forms of black Phosphorus. It has rhombohedral crystals or opaque monoclinic. It does not get oxidised in the air.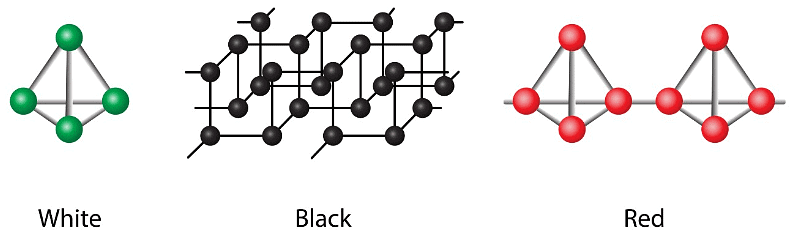
Different Compounds of Phosphorus
Compounds of Phosphorus and Oxygen
1. Phosphates are the compounds of derivatives of Phosphorus that are tetrahedral anions (PO3−4). Phosphates are the conjugate base of phosphoric acid. In nature, phosphates are present in the form of ATP (Adenosine Triphosphate). They contain P−O−P bonds.
2. Phosphorus (III) Oxide, having the chemical formula P4O6 is also known as tetraphosphorus hexoxide. It is the anhydride of P(OH)3. The structure of P4O6 resembles that of P4O10 without the terminal oxide groups.
3. Phosphoric acid or orthophosphoric acid or Phosphoric (v) acid is a weak acid with having the chemical formula H3PO4
Compounds of Phosphorus and Halogen
Some of the common compounds of Phosphorus and Halogens are PCl3,PCl5 and PF5
- PF5 is a colourless gas, and it has trigonal bipyramidal geometry.
- PCl5 is a colourless solid that can adopt the trigonal bipyramidal geometry when it is in the vapour phase.
- Phosphorus (III) halides: All four symmetrical trihalides of Phosphorus are in the form of gas PF3PF3 which is toxic in nature, PCl3 and PBr3 exists as yellowish liquids, and PI3 is in a solid-state. Phosphorus trichloride is a common reagent that is produced by chlorination of white Phosphorus:
P4+6Cl2 → 4PCl3 - Phosphides: Phosphides are formed from the reaction of metals with red Phosphorus. Binary phosphides consist of Phosphorus and one other element. The alkali metals and alkaline earth metals are capable of forming ionic compounds containing the phosphide ion. For example, Calcium phosphide (Ca3P2), Magnesium phosphide (Mg3P2), Aluminium phosphide (AlP), etc.
- Phosphines: It is a colourless gas having the smell of rotten eggs. The chemical formula of Phosphine is PH3.. It acts as a Lewis base by donating lone pair of electrons.
Uses of Phosphorus
- Red Phosphorus is mainly used for making matches. They are found on the sides of the matchbox, where matchsticks are struck to catch fire.
- White Phosphorus and zinc phosphate are widely used as a poison for rats.
- Phosphorus is used in making an alloy, ‘phosphor bronze’, which is an alloy of copper and tin-containing Phosphorus.
- Phosphorus compounds are significantly used as fertilisers like ammonium phosphate.
- In steel producing industries, Phosphorus is a vital element.
- Some detergents have Phosphate as an ingredient, but nowadays, this practice is not availed.
Phosphorus Cycle
- The phosphorus cycle is a biogeochemical cycle that deals with the movement of Phosphorus through the lithosphere, hydrosphere, as well as biosphere. The atmosphere does not play a significant role in the movement of Phosphorus, because phosphorus and phosphorus-based compounds are generally solids at the typical ranges of temperature and pressure found on the Earth.
- Therefore, the phosphorus cycle should be seen from the whole Earth system and then specifically is focused on the cycle in terrestrial and aquatic systems. With time, the amount of Phosphorus in the Earth is reducing and becomes less available to plants over thousands of years. A low concentration of Phosphorus in soil has several ill effects like it reduces plant growth and slows soil microbial growth.
- In nature, Phosphorus is found mostly in the form of phosphate ions. These compounds are found in sedimentary rocks. In the soil, Phosphate compounds can be taken up by plants and, from there, transferred to the herbivorous animals that eat these plants. When these plants and animals excrete wastes or die, phosphates may be decomposed and taken up by detritivores or returned to the soil.
- Phosphate or Phosphorus-containing compounds may also be carried in surface runoff to rivers, lakes, ponds, and oceans, where they are ingested by aquatic organisms. When these phosphorus-containing compounds from the bodies or wastes of marine or aquatic organisms sink to the floor of the ocean, they form new sedimentary layers, and this entire process takes thousands of years.
Summary
- Phosphorus is a highly reactive element and is present in different allotropic forms in nature as Red Phosphorus, White Phosphorus, and Black Phosphorus. In minerals, Phosphorus is found in the form of Phosphates which is also present in the body of living beings. Phosphorus forms several compounds with other elements like oxygen, nitrogen, hydrogen, sulphur, and halogens. Some of the compounds of Phosphorus include Phosphates, Phosphorus pentoxide, Phosphorus trichloride, Phosphorus pentafluoride, phosphides, phosphines, Phosphorus oxoacids, and many more.
- Phosphorus with oxygen forms compounds like Phosphate, Phosphoric acid, Oxides of Phosphorus, etc. At the same time, Phosphorus with halogens forms Phosphorus trihalides and Phosphorus pentahalides. Along with inorganic compounds, organic compounds are also produced from Phosphorus. Among these, many of them are useful for life, but some of them are very toxic. Fluorophosphate esters are one of the most potent neurotoxins. Due to their toxic behaviour, a wide range of organophosphorus compounds are used as pesticides.
|
129 videos|60 docs|24 tests
|




















