Sizes of Particles & their Properties | Chemistry for Grade 10 PDF Download
The Nano Scale
- Particles can be placed into one of three categories according to their diameter:
- Coarse particles (also called particulate-matter or dust)
- Fine particles
- Nanoparticles
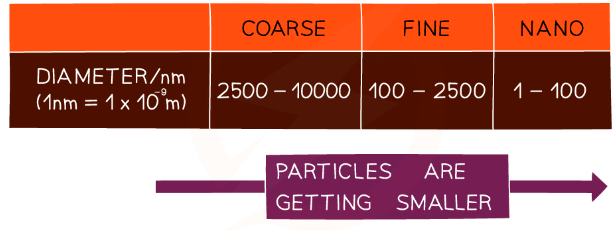 The diameter in nanometres used to classify particles
The diameter in nanometres used to classify particles
- Nanoparticles are between 1 and 100 nanometres in size and usually contain only a few hundred atoms
- Atoms and simple molecules are around 100 times larger than this
- Nanoparticles are much smaller than fine particles which have diameters of between 100 and 2500 nm
- The research into the production and application of nanoparticles is called nanoscience
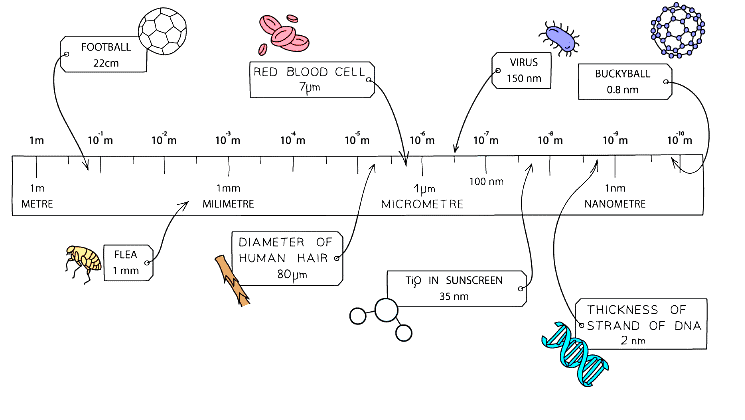 Diagram showing the size of nanoparticles relative to other objects and substances
Diagram showing the size of nanoparticles relative to other objects and substances
Exam Tip
1 nanometre = 1 x 10-9 m = 0.000 000 001 m.
Surface to Volume Ratio
- One of the most interesting features of nanoparticles is their very high surface area to volume ratio
- As particles decrease in size, their surface area increases in relation to their volume
As the side of a cube decreases by a factor of 10, the surface area to volume ratio increases by a factor of 10
This is why nanoparticles may have properties different from those for the same materials in bulk
It may also mean that smaller quantities are needed to be effective than for materials with normal particle sizes
Fullerenes (nanoparticles made of carbon) behave very differently to larger compounds of carbon like diamond and graphite
The surface area to volume ratio is an important feature in catalysis and surface chemistry
The higher the ratio then the more surface area is available for reaction, hence the better the catalyst
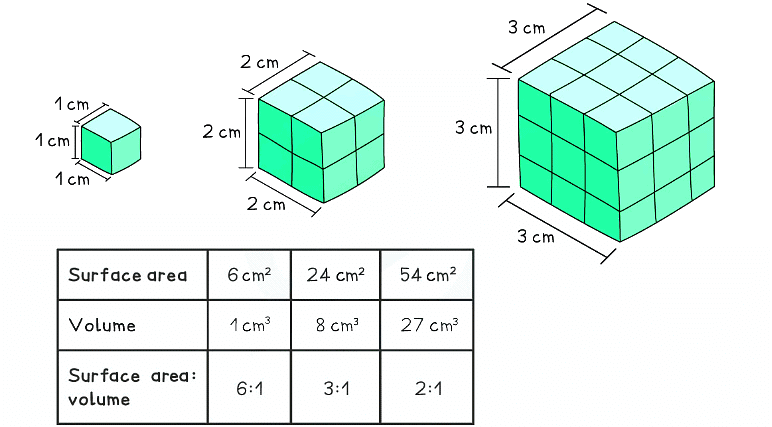 This diagram shows the surface area to volume ratio of three different sizes cubes
This diagram shows the surface area to volume ratio of three different sizes cubes
Exam Tip
Nanoparticles display different properties to the same element in bulk form due to their high surface to volume ratio.
|
75 videos|131 docs|24 tests
|















