Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Concentrations of Solutions
Concentrations of Solutions | Chemistry for Grade 10 PDF Download
Expressing Concentration
- A solid substance that dissolves in a liquid is called a solute, the liquid is called a solvent and the two when mixed together form a solution
- Most chemical reactions occur between solutes which are dissolved in solvents, such as water or an organic solvent
- Concentration simply refers to the amount of solute there is in a specific volume of the solvent
- The greater the amount of solute in a given volume, the greater the concentration
- A general formula for concentration is thus:
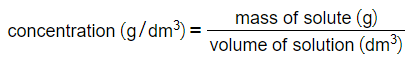
- Concentration can be measured in grams per cubic decimetre
- 1 decimetre cubed (dm3) = 1000 cm3
- 1 decimetre cubed (dm3) is the same as 1 litre
- You may be given data in a question which needs to be converted from cm3 to dm3 or the other way around
- To go from cm3 to dm3 :
- Divide by 1000
- To go from dm3 to cm3 :
- Multiply by 1000
- To go from cm3 to dm3 :
Calculating Concentration in Mass per Volume
- To calculate the concentration of a solution in terms of mass per unit volume, you need to
- Identify the solute and solvent
- Convert the volume units into decimetres cubed
- Divide the mass of the solute by the volume of the solution in decimetres cubed
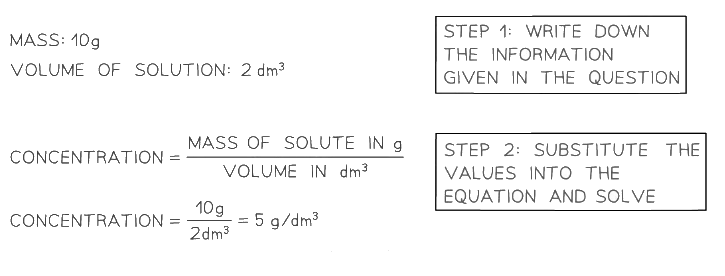
Worked Example
A student dissolved 10 g of sodium hydroxide, NaOH, in 2 dm3 of distilled water. Calculate the concentration of the solution.Exam Tip
Be careful when doing volume unit conversions as it is easy to multiply instead of dividing by 1000 and vice-versa. Always ask yourself - is the result going to be a bigger or smaller number than I started with? Do I get more or fewer cubic decimetres when I convert from cubic centimetres?
The document Concentrations of Solutions | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches