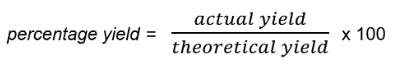Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Percentage Yield
Percentage Yield | Chemistry for Grade 10 PDF Download
Obtaining Calculated Masses: Basics
- Yield is the term used to describe the amount of product you get from a reaction
- In practice, you never get 100% yield in a chemical process for several reasons
- These include:
- Some reactants may be left behind in the equipment
- The reaction may be reversible and in these reactions a high yield is never possible as the products
- are continually turning back into the reactants
- Some products may also be lost during separation and purification stages such as filtration or distillation
- There may be side reactions occurring where a substance reacts with a gas in the air or an impurity in one of the reactants
- Products can also be lost during transfer from one container to another
Actual & Theoretical Yield
- The actual yield is the recorded amount of product obtained
- The theoretical yield is the amount of product that would be obtained under perfect practical and chemical conditions
- It is calculated from the balanced equation and the reacting masses
- The percentage yield compares the actual yield to the theoretical yield
- For economic reasons, the objective of every chemical producing company is to have as high a percentage yield as possible to increase profits and reduce costs and waste
Exam Tip
Although it’s very rare that they are equal, an efficient and well managed chemical process will produce an actual yield that is close to the theoretical yield.
Calculating Percentage Yield
- The percentage yield is a good way of measuring how successful a chemical process is
- There are often several methods of creating a compound and each method is called a reaction pathway
- Reaction pathways consist of a sequence of reactions which must occur to produce the required product
- Companies often investigate and try out different reaction pathways and these are then compared and evaluated so that a manufacturing process can be chosen
- The percentage yield of each pathway is a significant factor in this decision making process
- The equation to calculate the percentage yield is:

Worked Example
Copper(II) sulfate may be prepared by the reaction of dilute sulfuric acid on copper(II) oxide.A student prepared 1.6 g of dry copper(II)sulfate crystals.
Calculate the percentage yield if the theoretical yield is 2.0 g.
- Actual yield of copper(II)sulfate = 1.6 g
- Percentage yield of copper(II)sulfate = (1.6 / 2.0) x 100
- Percentage yield = 80%
Exam Tip
The actual yield and the % yield can be determined by experiment only, while the theoretical yield can be calculated assuming there is 100% conversion of reactants to products.
The document Percentage Yield | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
78 videos|87 docs|11 tests
|
Related Searches