Grade 10 Exam > Grade 10 Notes > Physics for Grade 10 > Atomic Structure
Atomic Structure | Physics for Grade 10 PDF Download
| Table of contents |

|
| Introduction |

|
| Parts of the Atom |

|
| Electron Structure |

|
| Electrons & Protons |

|
Introduction
- Atoms are the building blocks of all matter
- They are incredibly small, with a radius of only 1 × 10-10 m
- This means that about one hundred million atoms could fit side by side across your thumbnail
- Atoms have a tiny, dense nucleus at their centre, with electrons orbiting around the nucleus
- The radius of the nucleus is over 10,000 times smaller than the whole atom, but it contains almost all of the mass of the atom
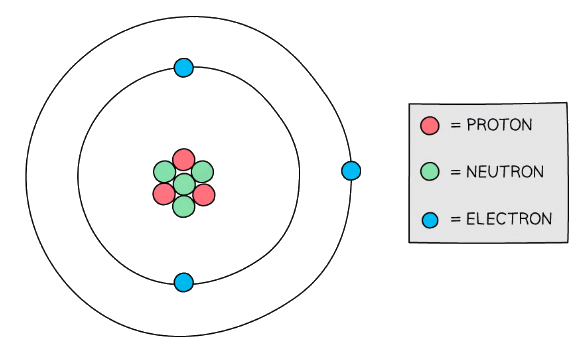 Diagram showing the structure of a Lithium atom. If drawn to scale then the electrons would be around 100 metres away from the nucleus!
Diagram showing the structure of a Lithium atom. If drawn to scale then the electrons would be around 100 metres away from the nucleus!
Parts of the Atom
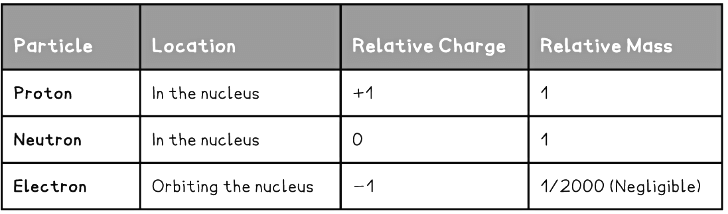
- The nucleus contains:
- Protons - positively charged particles with a relative atomic mass of one unit
- Neutrons – no charge, and also with a relative atomic mass of one unit
- Almost all of the atom is empty space, but moving around the nucleus there are:
- Electrons – negative charge with almost no mass (1/2000 the mass of a proton or neutron)
- The properties of each of the particles are shown in the table below:
Tip: There are many different models of the atom. As you progress through the topic you will discover that the atom can be described in many different ways, such as the Plum Pudding Model that is covered later, but for your exam, make sure to only use the model and descriptions described here!
Be careful with your terminology:
- Atom = nucleus (proton and neutron) and electrons
- Nucleus = protons and neutrons at the centre of the atom
Electron Structure
- Electrons in an atom orbit around the nucleus at particular distances, known as energy levels
- A certain number of electrons can occupy each energy level
- For example, only two electrons can orbit in the first energy level
- Only eight electrons can fit in the second energy level, and eight in the third as well
- The higher the energy level, the further the distance of the electron from the nucleus
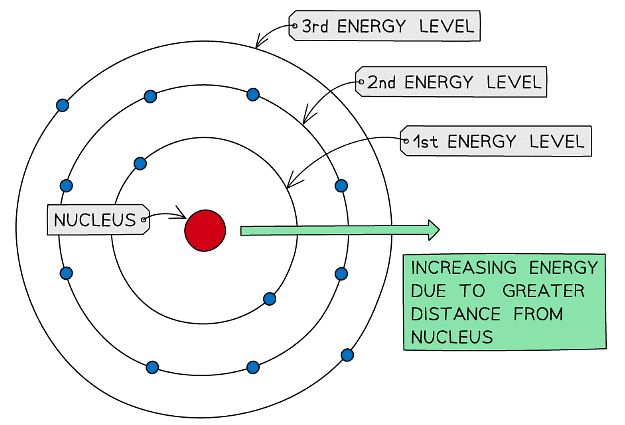 In this diagram the first two energy levels are full. Electrons further from the nucleus have more energy
In this diagram the first two energy levels are full. Electrons further from the nucleus have more energy - Like moving up a ladder, electrons in higher energy levels have greater potential energy because they have more distance between them and the nucleus
Tip: If you are studying for your Chemistry GCSE then you will need to know the number of electrons that fit into the different energy levels. They may also be called electron shells.
Electrons & Protons
- Although atoms contain particles of different charge, the total charge within an atom is zero
- This is because the number of electrons is equal to the number of protons
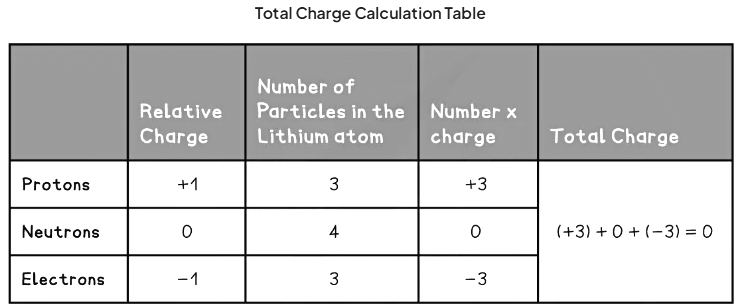
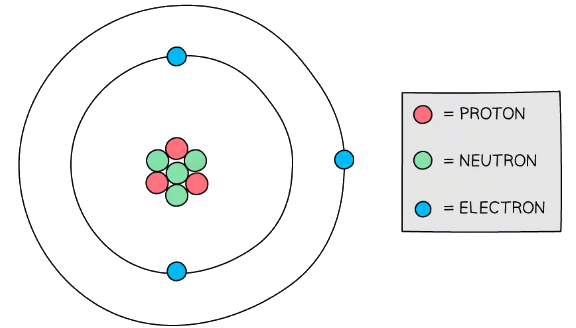 A Lithium atom has three protons, four neutrons and three electrons
A Lithium atom has three protons, four neutrons and three electrons
- This is because the number of electrons is equal to the number of protons
- The following table sets out the calculation of the total charge in the Lithium atom:
- If an atom loses electrons, then it is said to be ionised
Example: A nucleus of carbon-12 is shown below.
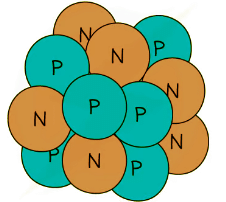
How many electrons are there in an atom of carbon-12?
Step 1: Count the number of protons in the carbon nucleus
- There are 6 protons in the carbon atom
Step 2: Determine the number of electrons
- Remember, the number of electrons in an atom is equal to the number of protons
- Therefore there must be 6 electrons in the carbon atom
The document Atomic Structure | Physics for Grade 10 is a part of the Grade 10 Course Physics for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
122 videos|149 docs|38 tests
|
Related Searches















