Grade 10 Exam > Grade 10 Notes > Physics for Grade 10 > Isotopes
Isotopes | Physics for Grade 10 PDF Download
| Table of contents |

|
| Isotopes |

|
| Tip |

|
| Differences Between Isotopes |

|
| Solved Example |

|
Isotopes
- Although the number of protons in a particular element is always the same, the number of neutrons can be different
- Isotopes are atoms of the same element that have an equal number of protons but a different number of neutrons
- In the diagram below are three isotopes of Hydrogen:
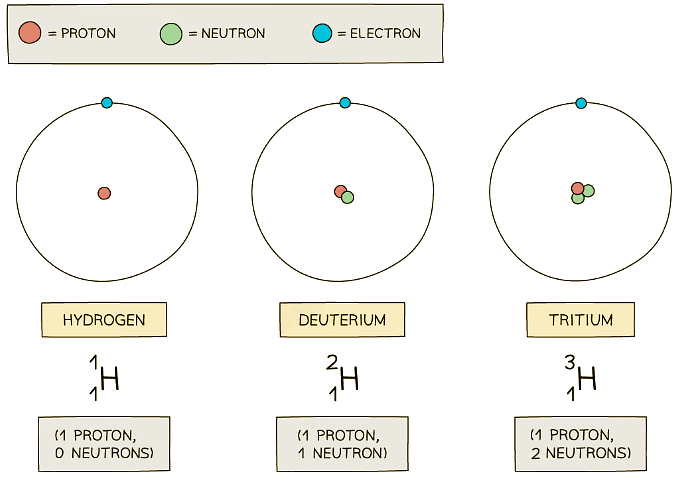 Hydrogen has three isotopes, each with a different number of neutrons
Hydrogen has three isotopes, each with a different number of neutrons - Isotopes occur naturally, but some are more rare than others
- For example, about 2 in every 10,000 Hydrogen atoms is Deuterium
- Tritium is even more rare (about 1 in every billion billion hydrogen atoms)
Tip
This topic is also covered in Chemistry, although some of the terminologies may be a little different. However, in Physics you must refer to neutrons when explaining isotopes.
Differences Between Isotopes
- The number of neutrons in an atom does not affect the chemical properties of an atom, such as its charge, but only its mass
- This is because neutrons have no charge but do have mass
- In the periodic table, the mass number of Chlorine is often given as 35.5
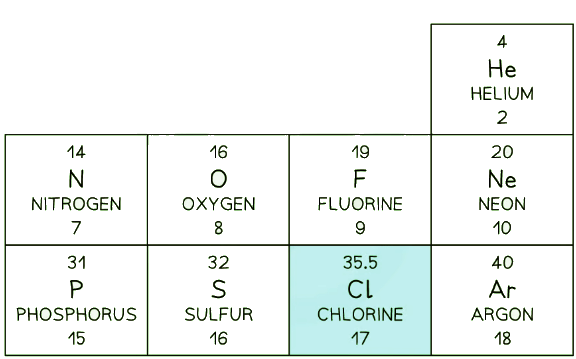 This section of a periodic table shows Chlorine as having a mass number of 35.5, but other elements have an integer mass number
This section of a periodic table shows Chlorine as having a mass number of 35.5, but other elements have an integer mass number - The mass number of Chlorine is given as 35.5 because it has roughly equal numbers of isotopes with a mass number of 35, and of 36
- The number of electrons and protons in different isotopes remains the same
- Isotopes tend to be more unstable due to the imbalance of protons and neutrons
Solved Example
Example: State the number of protons, neutrons and electrons in Chlorine-35 and Chlorine-36 atoms.
Step 1: Determine the number of protons
- The atomic number is the number of protons
- Both Chlorine-35 and Chlorine-36 have 17 protons
Step 2: Determine the number of neutrons
- The mass number is the number of protons and neutrons
- Chlorine-35 neutrons: 35 - 17 = 18 neutrons
- Chlorine-36 neutrons: 36 - 17 = 19 neutrons
Step 3: Determine the number of electrons
- The number of electrons is equal to the number of protons
- Both Chlorine-35 and Chlorine-36 have 17 electrons
The document Isotopes | Physics for Grade 10 is a part of the Grade 10 Course Physics for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
122 videos|152 docs|40 tests
|
Related Searches














