Grade 10 Exam > Grade 10 Notes > Physics for Grade 10 > Alpha, Beta & Gamma Decay
Alpha, Beta & Gamma Decay | Physics for Grade 10 PDF Download
| Table of contents |

|
| Alpha Decay |

|
| Decay Equations |

|
| Beta Decay |

|
| Gamma Decay |

|
Alpha Decay
- During alpha decay an alpha particle is emitted from an unstable nucleus
- A completely new element is formed in the process
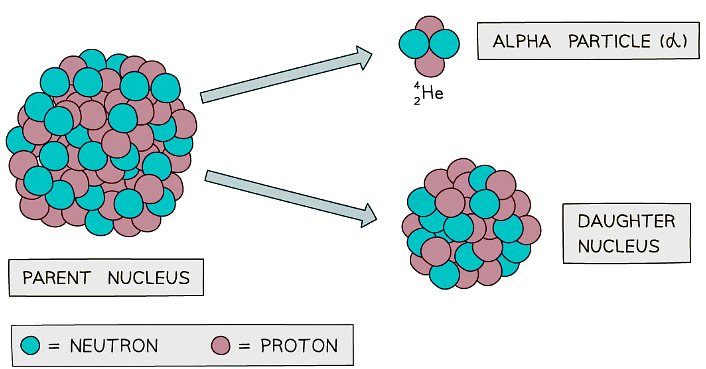 Alpha decay usually happens in large unstable nuclei, causing the overall mass and charge of the nucleus to decrease
Alpha decay usually happens in large unstable nuclei, causing the overall mass and charge of the nucleus to decrease - An alpha particle is a helium nucleus
- It is made of 2 protons and 2 neutrons
- When the alpha particle is emitted from the unstable nucleus, the mass number and atomic number of the nucleus changes
- The mass number decreases by 4
- The atomic number decreases by 2
- The charge on the nucleus also decreases by 2
- This is because protons have a charge of +1 each
Decay Equations
- The process of alpha decay can be shown as a decay equation
- A decay equation is similar to a chemical reaction equation
- The particles present before the decay are shown before the arrow
- The particles produced in the decay are shown after the arrow
- During decay equations, the sum of the mass and atomic numbers before the reaction must be the same as the sum of the mass and atomic numbers after the reaction
- The following equation shows Polonium-212 undergoing alpha decay
- It forms Lead-208 and an alpha particle
- An alpha particle can also be written as a helium nucleus (Symbol He)
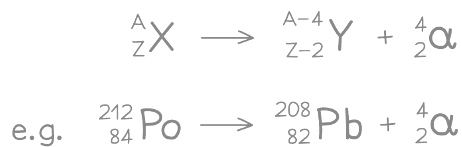 The polonium nucleus emits an alpha particle, causing its mass and charge to decrease. This means it changes into a new element
The polonium nucleus emits an alpha particle, causing its mass and charge to decrease. This means it changes into a new element
Tip: It is easy to forget that an alpha particle is a helium nucleus. The two are interchangeable, so don’t be surprised to see either used in the exam. You are not expected to know the names of the elements produced during radioactive decays, but you do need to be able to calculate the mass and atomic numbers by making sure they are balanced on either side of the reaction.
Question for Alpha, Beta & Gamma Decay
Try yourself:A nucleus with 84 protons and 126 neutrons undergoes alpha decay. It forms lead, which has the element symbol Pb.
Which of the isotopes of lead pictured is the correct one formed during the decay?
Which of the isotopes of lead pictured is the correct one formed during the decay?
View Solution
Beta Decay
- During beta decay, a neutron changes into a proton and an electron
- The electron is emitted and the proton remains in the nuclei
- A completely new element is formed because the atomic number changes
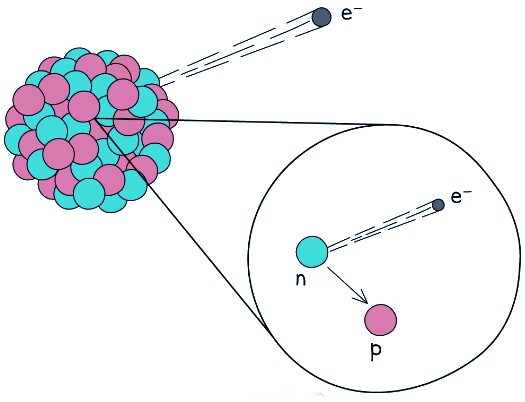 Beta decay often happens in unstable nuclei that have too many neutrons. The mass number stays the same, but the atomic number increases by one
Beta decay often happens in unstable nuclei that have too many neutrons. The mass number stays the same, but the atomic number increases by one - A beta particle is a high-speed electron
- It has a mass number of 0
- This is because the electron has a negligible mass, compared to neutrons and protons
- Therefore, the mass number of the decaying nuclei remains the same
- Electrons have an atomic number of -1
- This means that the new nuclei will increase its atomic number by 1 in order to maintain the overall atomic number before and after the decay
- The following equation shows carbon-14 undergoing beta decay
- It forms nitrogen-14 and a beta particle
- Beta particles are written as an electron in this equation
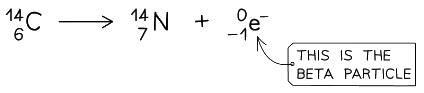 The carbon nucleus emits a beta particle, causing its charge to increase. This means it changes into a new element
The carbon nucleus emits a beta particle, causing its charge to increase. This means it changes into a new element
Tip: There is a second form of beta decay during which a proton changes into a neutron. This is called beta-plus decay - you might come across it while revising, but you don't need to know about it for your exam. Only use the information here for your GCSE.
Question for Alpha, Beta & Gamma Decay
Try yourself:A nucleus with 11 protons and 13 neutrons undergoes beta decay. It forms magnesium, which has the element symbol Mg.
Which is the correct isotope of magnesium formed during the decay?
Which is the correct isotope of magnesium formed during the decay?
View Solution
 |
Download the notes
Alpha, Beta & Gamma Decay
|
Download as PDF |
Download as PDF
Gamma Decay
- During gamma decay, a gamma ray is emitted from an unstable nucleus
- The process that makes the nucleus less energetic but does not change its structure
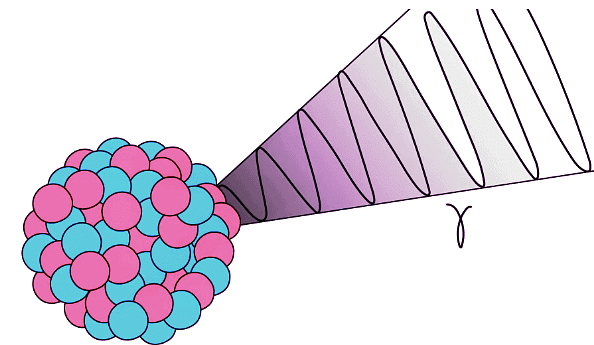 Gamma decay does not affect the mass number or the atomic number of the radioactive nucleus, but it does reduce the energy of the nucleus
Gamma decay does not affect the mass number or the atomic number of the radioactive nucleus, but it does reduce the energy of the nucleus - The gamma ray that is emitted has a lot of energy, but no mass or charge
- Here is an example of Uranium-238 undergoing gamma decay
- Notice that the mass number and atomic number of the unstable nuclei remains the same during the decay
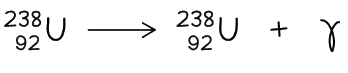 Although the Uranium nucleus is unchanged in structure, its energy reduces during gamma decay
Although the Uranium nucleus is unchanged in structure, its energy reduces during gamma decay
- Notice that the mass number and atomic number of the unstable nuclei remains the same during the decay
The document Alpha, Beta & Gamma Decay | Physics for Grade 10 is a part of the Grade 10 Course Physics for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
124 videos|149 docs|37 tests
|
Related Searches
















