Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Neutralisation of Acids and Salt Production
Neutralisation of Acids and Salt Production | Chemistry for Grade 10 PDF Download
Neutralisation
- Neutralisation is the reaction of an acid with a base that results in the pH moving towards 7.
- It is a useful process that occurs in everyday life such as in the treatment of acid indigestion and the treating of acidic soil by adding lime.
- Neutralisation also moves the pH of an alkali down towards seven.
- Several different bases can neutralise acids, and water is always produced as a result of these reactions.
Equations for neutralisation
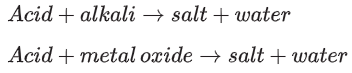
Metal oxides and alkalis are two types of base. Basic substances neutralise acids, resulting in the pH of the acid increasing towards 7, and water being produced. A soluble base dissolves in water to form an alkaline solution.
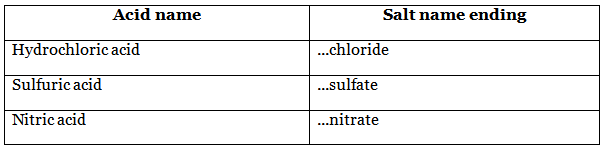
Naming salts
To name the salt, the metal ion from the alkali (or base) replaces the hydrogen ion from the acid - (alkali to front, acid to back).
For example:
hydrochloric acid + sodium hydroxide → sodium chloride + water
During neutralisation the H+ ion from the acid joins with the OH- ion from the alkali. This is why water is formed in these reactions.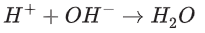 This is true for all neutralisation reactions.
This is true for all neutralisation reactions.
Acids can be neutralised by metal carbonates
In the neutralisation reaction between an acid and a metal carbonate, there are three products. The hydrogen ions (H+) from the acid react with the carbonate ions (CO32-) to form water and carbon dioxide gas. A salt is also produced.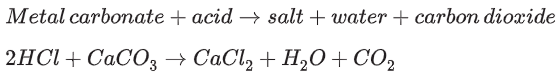
- The salt is named in the same way as before, taking the metal's name from the carbonate and the ending from the type of acid used.
- Carbon dioxide can be tested for using lime water (turns from colourless to chalky white).
- When insoluble metal carbonates and insoluble metal oxides are used to produce soluble salts, excess base is added to the acid.
- The mixture can then be filtered to remove unreacted metal carbonate or metal oxide.
- The filtrate can then be evaporated to leave the salt produced.
The document Neutralisation of Acids and Salt Production | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches




















