Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Strong & Weak Acids
Strong & Weak Acids | Chemistry for Grade 10 PDF Download
Higher Tier Only
- Acids can be either strong or weak, depending on how many ions they produce when they dissolve in water
- When added to water, acids ionise or dissociate to produce H+ ions:
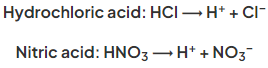
- Strong acids such as HCl and H2SO4 dissociate completely in water, producing solutions with a high concentration of H+ ions and thus a very low pH
- Weak acids such as ethanoic acid, CH3COOH, and hydrofluoric acid, HF, only partially ionise in water, producing solutions of pH values between 4 – 6
- This data is summarised in the table below:
Strong & Weak Acids Table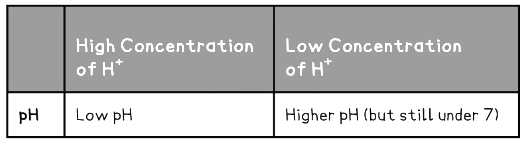
- For weak acids, there is an equilibrium set-up between the molecules and their ions once they have been added to water
- Propanoic acid for example dissociates as follows:

- The ⇌ symbol indicates that the process is reversible, as the products can react together forming the original reactants
- The equilibrium lies to the left, meaning there is a high concentration of intact acid molecules and therefore a low concentration of ions in solution, hence the pH is that of a weak acid and closer to 7 than a strong acid.
Exam Tip
Careful: The terms strong and weak refer to the ability to dissociate whereas the term concentration refers to the amount of acid present in solution. A dilute solution of a strong acid can have a lower pH than a concentrated solution of a weak acid, due to the stronger acid undergoing complete dissociation.
Hydrogen Ion Concentration
- A concentrated solution of either an acid or a base is one that contains a high number of acid or base molecules per dm3 of solution so would produce pH values below 4 and above 10
- A dilute acid or base solution is therefore one that has much fewer acid or base molecules per dm3 of solution, hence the pH value would lie between 5 and 9
- It does not necessarily mean that the acid or base is strong as it may be made from a weak acid or base which does not dissociate completely but a lot of it was added to the solution
- For example, a dilute solution of HCl will be more acidic than a concentrated solution of ethanoic acid, since most of the HCl molecules dissociate but very few of the CH3COOH molecules do
Exam Tip
Remember concentration describes the total number of acid molecules added to the solution but does not consider those that dissociated. This is measured using the pH scale.
Relative Acidity
- We have already seen that pH is a measure of the concentration of H+ ions in solution
- The pH scale is logarithmic, meaning that each change of 1 on the scale represents a change in concentration by a factor of 10
- Therefore an acid with a pH of 3 has ten times the concentration of H+ ions than an acid of pH 4
- An acid with a pH of 2 has 10 x 10 = 100 times the concentration of H+ ions than an acid with a pH of 4
- From this we can summarise that for two acids of equal concentration, where one is strong and the other is weak, then the strong acid will have a lower pH due to its capacity to dissociate more and hence put more H+ ions into solution than the weak acid
Exam Tip
Acid strength indicates the proportion of acid molecules that dissociate while concentration is a measure of how much acid there is per unit volume of water.
The document Strong & Weak Acids | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches




















