Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Half Equations in Electrolysis
Half Equations in Electrolysis | Chemistry for Grade 10 PDF Download
| Table of contents |

|
| Higher Tier Only |

|
| Electrolysis of molten lead(II)bromide |

|
| Electrolysis of molten aluminium oxide |

|
| Electrolysis of aqueous solutions |

|
Higher Tier Only
- In electrochemistry we are mostly concerned with the transfer of electrons, hence the definitions of oxidation and reduction are applied in terms of electron loss or gain rather than the addition or removal of oxygen
- Oxidation is when a substance loses electrons and reduction is when a substance gains electrons
- As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral substances
- These are then discharged as products at the electrodes
- At the anode, negatively charged ions lose electrons and are thus oxidised
- At the cathode, the positively charged ions gain electrons and are thus reduced
- This can be illustrated using half equations which describe the movement of electrons at each electrode
- It is important to make sure that the charges as well as the number of atoms/ions on each side of the equation are balanced
Electrolysis of molten lead(II)bromide
- In the electrolysis of molten lead(II) bromide the half equation at the negative electrode (cathode) is:
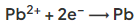
- At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions:
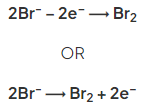
Electrolysis of molten aluminium oxide
- Aluminium ions are discharged at the negative electrode (cathode) and the aluminium is collected at the bottom of the cell:
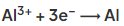
- At the positive electrode (anode) oxygen gas is produced:
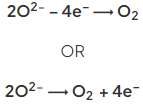
Electrolysis of aqueous solutions
- At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged and the half equation is:
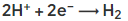
- When the metal is less reactive than hydrogen, the metal is discharged, e.g.:
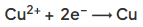
- At the positive electrode (anode), if a halide ion is present, the corresponding halogen is formed e.g.:
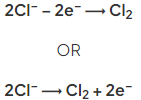
- When a halide ion is not present, oxygen is formed as hydroxide ions are discharged, e.g.:
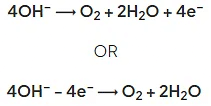
- Half equations illustrate the transfer of electrons during a chemical process as they provide a more detailed picture of the redox processes taking place
- Half equations combine to give the ionic equation for an electrolytic cell.
The example below illustrates how this is done for the sodium chloride:
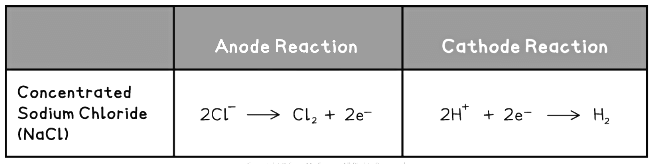 Electrode Half Equations Table 1
Electrode Half Equations Table 1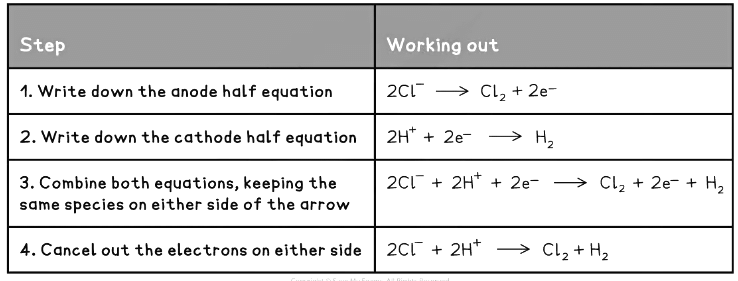 Electrode Half Equations Table 2
Electrode Half Equations Table 2
- The table below shows the half equations for a number of common electrolytes, dilute and concentrated where applicable:
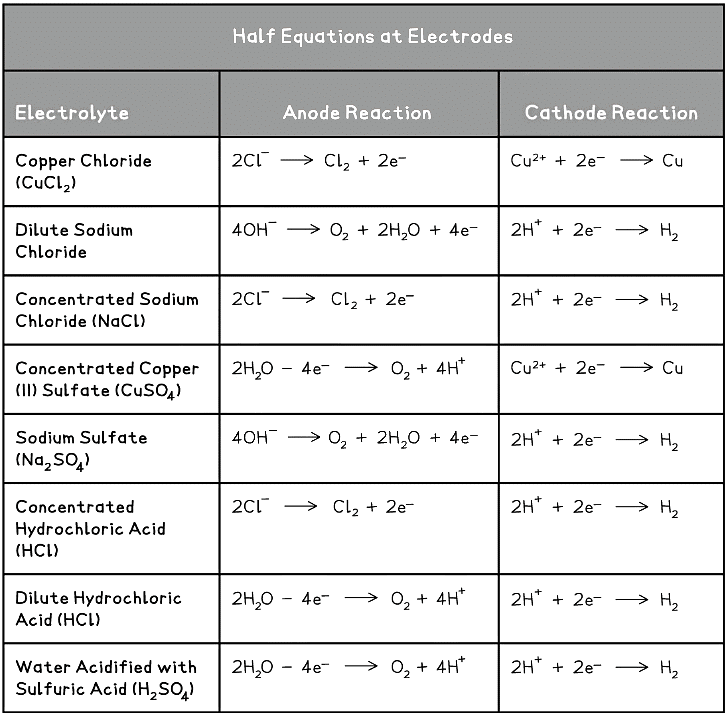
Exam Tip
Don't forget to make sure the charges are balanced within the equation!
The document Half Equations in Electrolysis | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches


















