Introduction: Organic Chemistry | Chemistry for Grade 10 PDF Download
| Table of contents |

|
| Organic Chemistry |

|
| Types of Formulae |

|
| Homologous Series |

|
| Composition of Crude Oil |

|
| Alkanes |

|
Organic Chemistry
- Organic chemistry is the chemistry of carbon compounds
- Carbon forms a vast number of compounds because it can form strong covalent bonds with itself
- This enables it to form long chains of carbon atoms, and hence an almost infinite variety of carbon compounds are known
- Carbon always forms four covalent bonds which can be single, double or triple bonds
- A functional group is a specific atom or group of atoms which confer certain physical and chemical properties onto the molecule
- Organic molecules are classified by the dominant functional group on the molecule
- Organic compounds with the same functional group, but a different number of carbon atoms, are said to belong to the same homologous series
- Every time a carbon atom is added to the chain, two hydrogen atoms are also added
- Organic compounds can be represented in a number of ways
Types of Formulae
General Formulae
- This type of formula tells you the composition of any member of a whole homologous series of organic compound
- For example, all of the alkanes have the general formula CnH2n+2
- This tells you that however many carbon atoms there are in the alkane, doubling this number and adding two will give you the number of hydrogen atoms present in the alkane
Displayed Formulae
- This shows the spatial arrangement of all the atoms and bonds in a molecule
Molecular Formulae
- This shows the actual number of each atom in a molecule, one molecule at a time
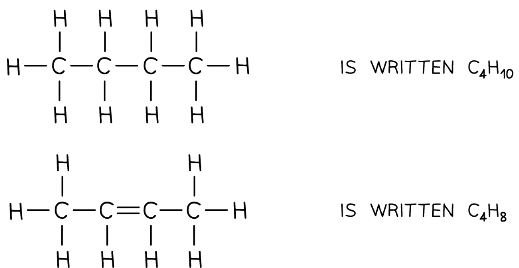 The displayed formulae and molecular formulae of butane and butene
The displayed formulae and molecular formulae of butane and butene
- Structural Formulae
- This gives enough information to make the structure clear, but most of the actual covalent bonds are omitted
- Only important bonds are shown, such as double and triple bonds
- Identical groups can be bracketed together
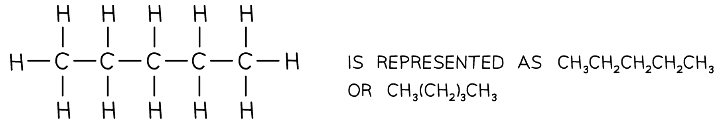 The displayed formula followed by the structural formula of pentane
The displayed formula followed by the structural formula of pentane
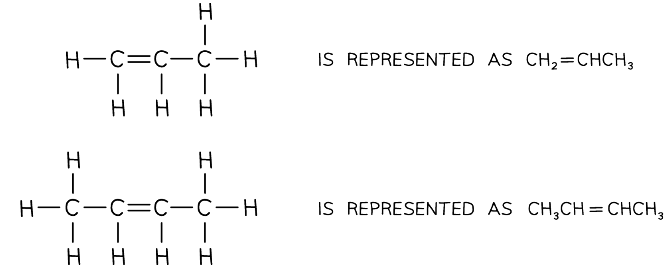 Representing structural formulae of two alkenes
Representing structural formulae of two alkenes
Homologous Series
- Things we can say about a homologous series:
- each member has the same functional group
- each member has the same general formula
- each member has similar chemical properties
- each subsequent member differs by -CH2 -
- members have gradually changing physical properties, for example, boiling point, melting point and density
- As a homologous series is ascended, the size of the molecule increases
- This has an effect on the physical properties, such as boiling point and density
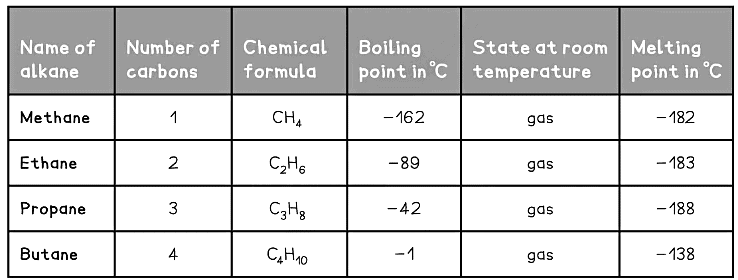 Homologous Series of Alkanes Table
Homologous Series of Alkanes Table
Composition of Crude Oil
- Crude oil is a finite resource which we find in the Earth's crust
- It is a complex mixture of compounds, mainly hydrocarbons, which also contains natural gas
- Hydrocarbons are compounds that are made of carbon and hydrogen atoms only
- The hydrocarbon molecules in crude oil consist of a carbon backbone which can be in a ring or chain, with hydrogen atoms attached to the carbon atoms
- The mixture contains molecules with many different ring sizes and chain lengths
- It is a thick, sticky, black liquid that is found in porous rock (under the ground and under the sea)
- Crude oil formed over millions of years from the effects of high pressures and temperatures on the remains of biomass (plants and animals), mainly plankton that was buried in mud
- It is being used up much faster than it is being formed, which is why we say crude oil is a finite resource
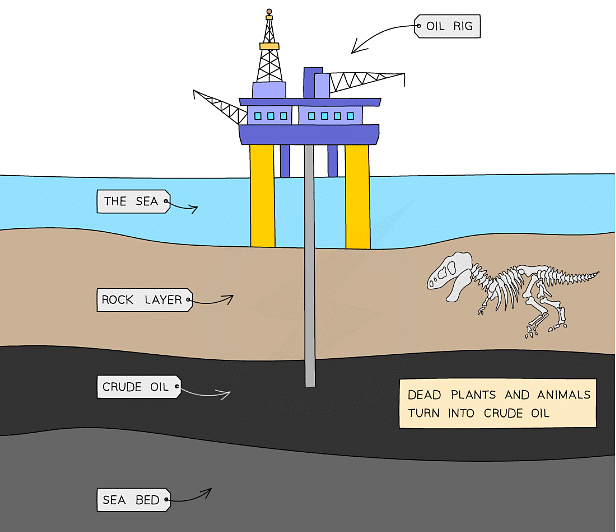 Crude oil found under the sea
Crude oil found under the sea
Exam Tip
Crude oil is also sometimes referred to as petroleum. Some fractions may have different names in the UK and the USA, e.g. gasoline is the name used in the USA for petrol. You may be asked to give a definition of the term hydrocarbon - be careful! You must say a compound which contains carbon and hydrogen atoms only. If you do not say only, then you will not get the mark.
Alkanes
- Alkanes are a group of saturated hydrocarbons
- The term saturated means that they only have single carbon-carbon bonds, there are no double bonds
- They form a homologous series which has the general formula CnH2n+2
- They are colourless compounds which have a gradual change in their physical properties as the number of carbon atoms in the chain increases
- Alkanes are generally unreactive compounds but they do undergo combustion reactions, can be cracked into smaller molecules and can react with halogens in the presence of light
- Methane is an alkane and is the major component of natural gas
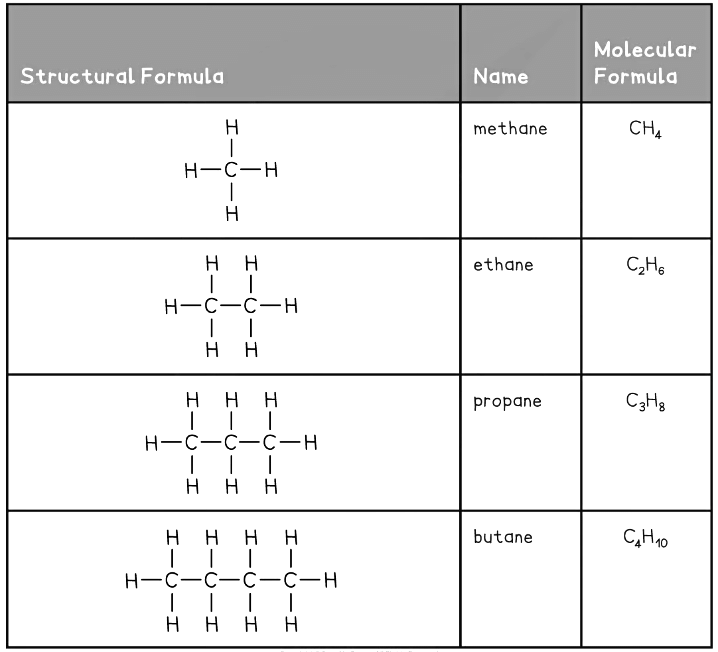 This table shows the first four members of the alkane homologous series
This table shows the first four members of the alkane homologous series
Exam Tip
For your exam, you need to be able to name, draw and give the appropriate formula for the first four alkanes.
|
75 videos|131 docs|24 tests
|














