Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Alcohols - Reactions of Alkenes & Alcohols
Alcohols - Reactions of Alkenes & Alcohols | Chemistry for Grade 10 PDF Download
| Table of contents |

|
| The Alcohol Functional Group |

|
| The First Four Alcohols |

|
| Production of Ethanol by Fermentation |

|
| Reactions of Alcohols |

|
The Alcohol Functional Group
- All alcohols contain the hydroxyl (-OH) functional group which is the part of alcohol molecules that is responsible for their characteristic reactions
- Alcohols are a homologous series of compounds that have the general formula CnH2n+1OH
- They differ by one -CH2 in the molecular formulae from one member to the next
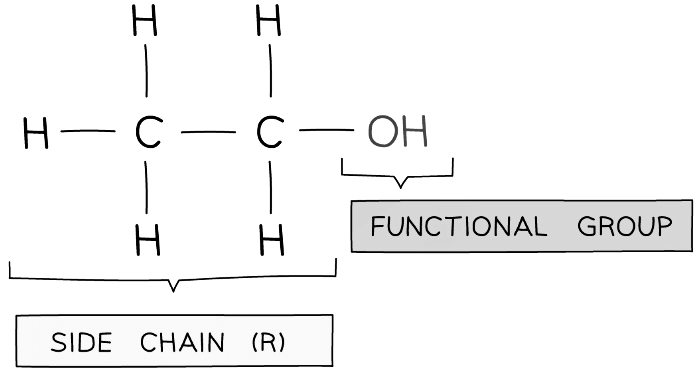 Diagram of the side chain and -OH group in ethanol which characterizes its chemistry
Diagram of the side chain and -OH group in ethanol which characterizes its chemistry
Exam Tip
Don’t confuse the -OH functional group with the hydroxide ion, OH–, they are not the same thing!
The First Four Alcohols
- The names and structures of the first four alcohols are shown below
- In terms of naming, the same system is used as for alkanes and alkenes, with the final ‘e’ being replaced with ‘ol’
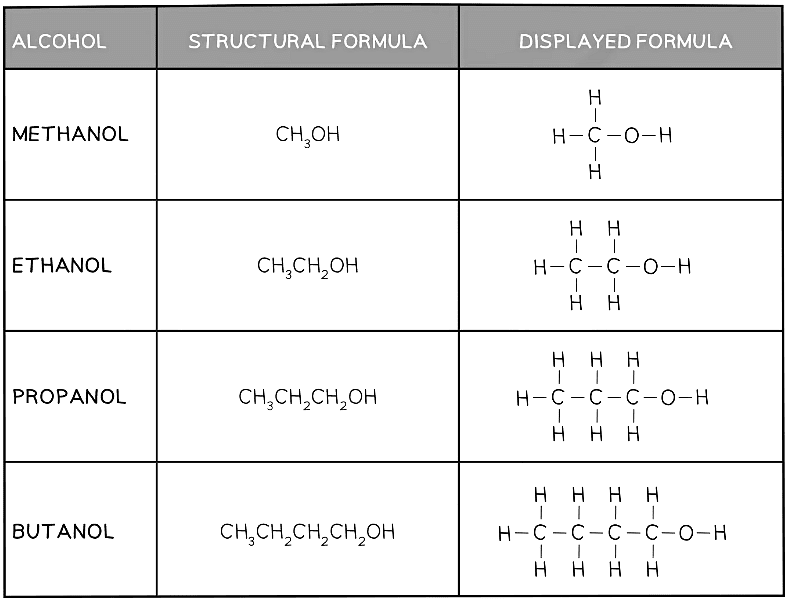 Table showing the formulae and structures of the first four alcohols
Table showing the formulae and structures of the first four alcohols
Exam Tip
It is standard practice to write the functional group on the end as it shows what the molecule is. E.g. Methanol is CH3OH, not CH4O.
Production of Ethanol by Fermentation
- Ethanol (C2H5OH) is one of the most important alcohols
- It is the type of alcohol found in alcoholic drinks such as wine and beer
- It is also used as fuel for cars and as a solvent
- It can be produced by fermentation where sugar or starch is dissolved in water and yeast is added
- The mixture is then fermented between 15 and 35°C with the absence of oxygen for a few days
- Yeast contains enzymes that break down sugar to glucose
- If the temperature is too low the reaction rate will be too slow and if it is too high the enzymes will become denatured
- The yeast respire anaerobically using the glucose to form ethanol and carbon dioxide:
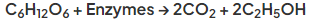
- The yeast are killed off once the concentration of alcohol reaches around 15%, hence the reaction vessel is emptied and the process is started again
- This is the reason that ethanol production by fermentation is a batch process
Exam Tip
Fermentation is an anaerobic process. Oxygen is not required for ethanol to be produced by fermentation.
Reactions of Alcohols
- Alcohols are colourless liquids that dissolve in water to form neutral solutions
- The first four alcohols are commonly used as fuels
- School laboratories use ethanol in spirit burners as it burns cleanly and without strong odours
- Methanol and ethanol are also used extensively as solvents
- This is because they can dissolve many substances that water cannot such as fats and oils, but can also dissolve most of the substances that water can
- Alcohols undergo combustion to form carbon dioxide and water
- The complete combustion of ethanol is as follows:
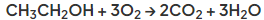
- Alcohols react with sodium metal to produce hydrogen gas and a metal salt
- The word equation for the reaction of methanol with sodium is:
sodium + methanol → sodium methoxide + hydrogen - Alcohols undergo oxidation to produce carboxylic acids, an organic acid
- This is what happens to wine when it is left open as the microbial oxidation of ethanol will produce a weak solution of a carboxylic acid called ethanoic acid, the same acid used in vinegar
- Bacteria in the air (acetobacter) use atmospheric oxygen from air to oxidise the ethanol in the wine:
ethanol + oxygen → ethanoic acid + water - The acidic, vinegary taste of wine which has been left open for several days is due to the presence of ethanoic acid
Exam Tip
You need to be able to write and balance equations for the combustion of alcohols.
The document Alcohols - Reactions of Alkenes & Alcohols | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches














