Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Carboxylic Acids
Carboxylic Acids | Chemistry for Grade 10 PDF Download
The Carboxylic Acid Functional Group
- Carboxylic acids are a homologous series of compounds that have the general formula of
CnH2n+1 COOH - They differ by one -CH2 in the molecular formulae from one member to the next
- They show a gradation in their physical properties:
- Boiling points increase with increased carbon chain length
- Viscosity increases with increased carbon chain length
- They have similar chemical properties
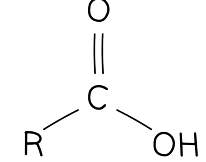 Diagram of the general structure of a carboxylic acid. The R- represents a varying hydrocarbon chain
Diagram of the general structure of a carboxylic acid. The R- represents a varying hydrocarbon chain
Exam Tip
The carbon atom in the -COOH functional group is counted as part of the molecule and not just the functional group. E.g. CH3CH2CH2COOH has 4 carbon atoms so is called butanoic acid, not propanoic acid.
The First Four Carboxylic Acids
- The names and structure of the first four carboxylic acids are shown below
- In terms of naming, the same system is used as for alkanes and alkenes, with the final ‘e’ being replaced with ‘oic’ and then the word acid
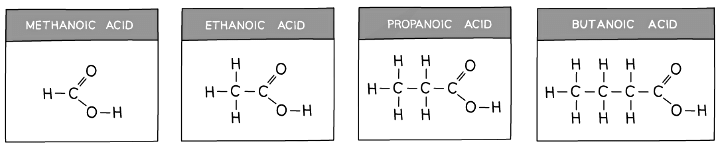 The structures and formulae of the first four carboxylic acids
The structures and formulae of the first four carboxylic acids
Reactions of Carboxylic Acids
- The carboxylic acids behave like other acids
- They react with metals to form a salt and hydrogen and with carbonates to form a salt, water and carbon dioxide gas
- They take part in neutralisation reactions to produce salt and water
- Ethanoic acid (also called acetic acid) is the acid used to make vinegar, which contains around 5% by volume ethanoic acid
- The salts formed by the reaction of carboxylic acids all end –anoate
- So methanoic acid forms a salt called methanoate, ethanoic a salt called ethanoate etc
- In the reaction with metals, a metal salt and hydrogen gas are produced
- For example in the reaction of ethanoic acid with magnesium, the salt magnesium ethanoate is formed:
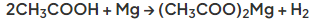
- In the reaction with hydroxides a salt and water are formed in a neutralisation reaction
- For example in reaction with potassium hydroxide the salt potassium propanoate is formed by reaction with propanoic acid:
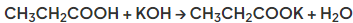
- In the reaction with carbonates a metal salt, water and carbon dioxide gas are produced
- For example in reaction with potassium carbonate the salt potassium butanoate is formed by reaction with butanoic acid:
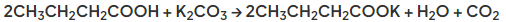
- Alcohols and carboxylic acids react to make esters in esterification reactions in the presence of an acid catalyst, usually concentrated sulfuric acid
- Esters are compounds with the functional group R-COO-R
- Esters are sweet smelling oily liquids used in food flavourings and perfumes
- They are volatile, meaning they vapourise easily
- Ethanoic acid will react with ethanol in the presence of concentrated sulfuric acid (catalyst) to form ethyl ethanoate:
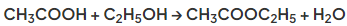
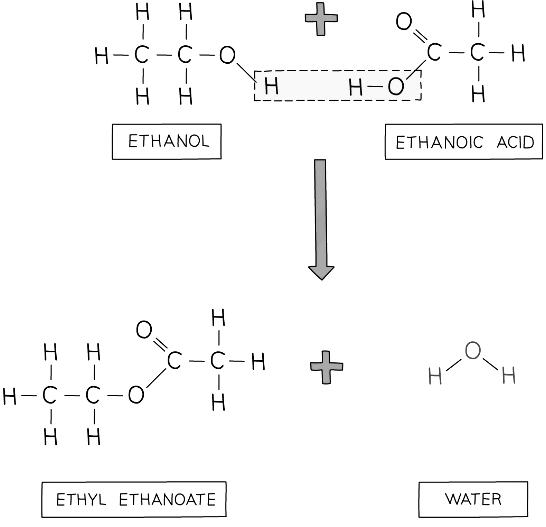 Diagram showing the formation of ethyl ethanoate
Diagram showing the formation of ethyl ethanoate
Exam Tip
You are not expected to be able to write balanced equations for the reactions of carboxylic acids- they are included here for background information.
The document Carboxylic Acids | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches




















