Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Condensation Polymerisation
Condensation Polymerisation | Chemistry for Grade 10 PDF Download
Higher Tier Only
- Condensation polymers are formed when two different monomers are linked together with the removal of a small molecule, usually water
- This is a key difference between condensation polymers and addition polymers:
- Addition polymerisation forms the polymer molecule only
- Condensation polymerisation forms the polymer molecule and one water molecule per linkage
- The monomers have two functional groups present, one on each end
- The functional groups at the ends of one monomer react with the functional group on the end of the other monomer, in so doing creating long chains of alternating monomers, forming the polymer
- Polyesters are formed from two different monomers and produce water
- For every ester linkage formed in condensation polymerisation, one molecule of water is formed from the combination of a proton (H+) and a hydroxyl ion (OH–)
- An example is terylene which is a polyester made from dicarboxylic acid monomers (a carboxylic with a -COOH group at either end) and diols (an alcohol with an -OH group at either end)
- Each -COOH group reacts with another -OH group on another monomer
- An ester linkage is formed with the subsequent loss of one water molecule per link
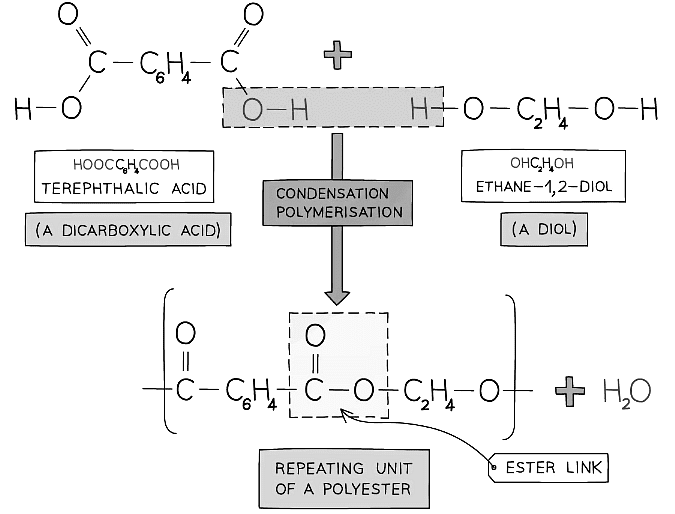 The condensation reaction in which the polyester terylene is produced
The condensation reaction in which the polyester terylene is produced
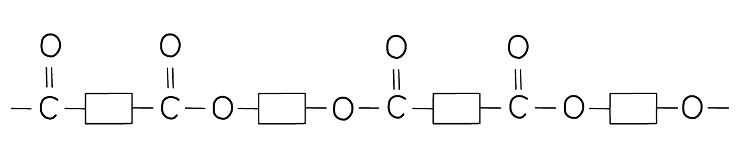
- The structure of terylene can be represented by drawing out the polymer using boxes to represent the carbon chains
- This can be done for all polyesters
 Diagram showing a section of the polyester terylene
Diagram showing a section of the polyester terylene
Exam Tip
Notice that the sequence of bonding in the polyester is the mirror image at either end of the link, NOT the link repetition due to the monomers containing the same functional group at either end.
The document Condensation Polymerisation | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
78 videos|87 docs|11 tests
|
Related Searches