Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Pure Substances
Pure Substances | Chemistry for Grade 10 PDF Download
Pure Substances
- In everyday language, we use the word pure to describe when something is natural or clean and to which nothing else has been added
- In chemistry, a pure substance may consist of a single element or compound which contains no other substances
- For example, a beaker of a sample of pure water contains only H2O molecules and nothing else
- If salt were added to the beaker then a mixture is produced
- A mixture consists of two or more elements or compounds that are physically mixed together, they are not chemically combined
- The chemical properties of the substances in a mixture remain unchanged
- Substances in mixtures can be separated by physical means
- Air for example is a mixture of nitrogen, oxygen and some other gases such as carbon dioxide and argon
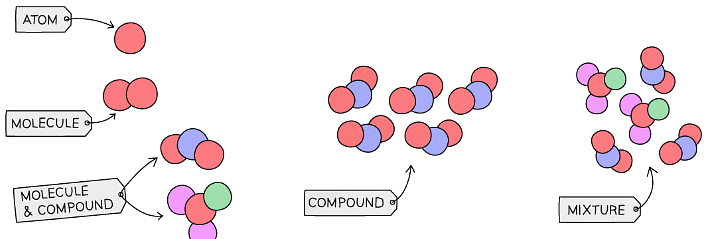 Diagram showing how to represent elements, compounds and mixtures using particle diagrams
Diagram showing how to represent elements, compounds and mixtures using particle diagrams
Distinguishing Purity
- Pure substances melt and boil at specific and sharp temperatures e.g. pure water has a boiling point of 100°C and a melting point of 0°C
- Mixtures have a range of melting and boiling points as they consist of different substances that tend to lower the melting point and broaden the melting point range
- Melting and boiling points data can therefore be used to distinguish pure substances from mixtures
- Melting point analysis is routinely used to assess the purity of drugs
- This is done using a melting point apparatus which allows you to slowly heat up a small amount of the sample, making it easier to observe the exact melting point
- This is then compared to data tables
- The closer the measured value is to the actual melting or boiling point then the purer the sample is
Cooling Curves
- The influence of impurities can be more clearly seen on a heating / cooling curve
- If the temperature of a liquid is measured as it cools and freezes the data can be used to produce a graph
- The following graph shows the cooling curve for a sample of a compound
- The horizontal part of the graph shows that the compound has a sharp melting point, so the compound is pure
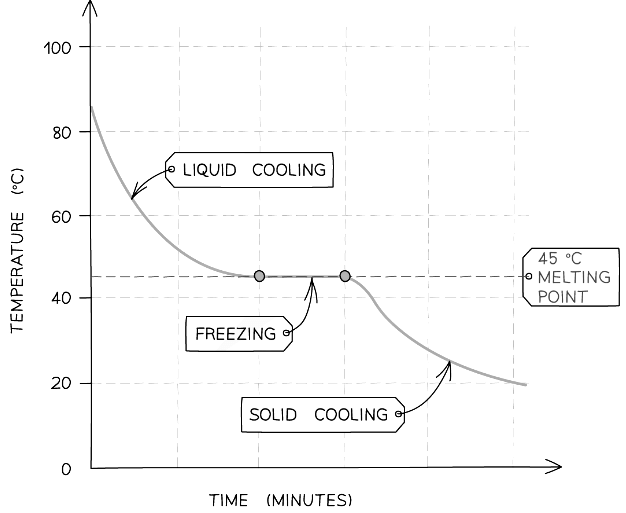 Cooling curve for a pure substance
Cooling curve for a pure substance
- An impure sample of the compound would produce a gradual decrease in temperature as it freezes, as shown in the graph below
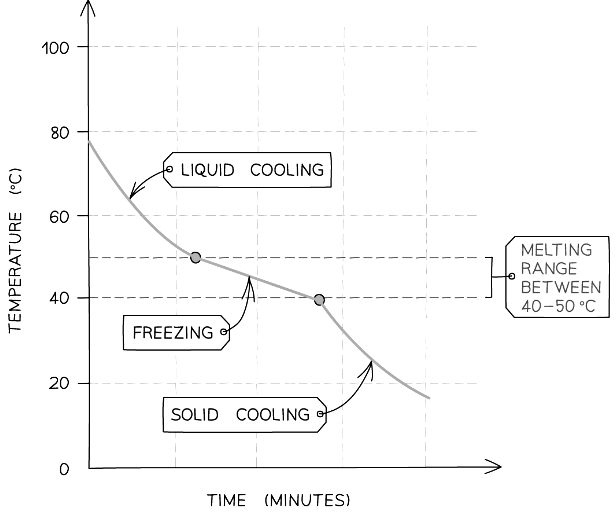 Cooling curve for an impure substance
Cooling curve for an impure substance
The document Pure Substances | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches















