Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Metal Hydroxides
Metal Hydroxides | Chemistry for Grade 10 PDF Download
About Metal Hydroxides
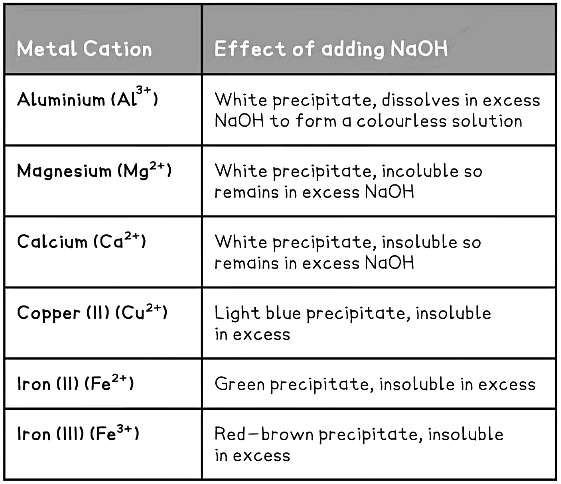
- Metal cations in aqueous solution can be identified by the colour of the precipitate they form on addition of sodium hydroxide
- If only a small amount of NaOH is used then normally the metal hydroxide precipitates
- In excess NaOH some of the precipitates may dissolve
- For this reason just a few drops of NaOH are added at first and very slowly
- If it is added too quickly and the precipitate is soluble in excess, then you run the risk of missing the formation of the initial precipitate which dissolves as quickly as it forms if excess solution is added
- A small amount is thus added, very gradually and any colour changes or precipitates formed are noted
- Then the NaOH is added in excess and the reaction is observed again
- Ca2+ and Mg2+ ions can be distinguished from Al3+ as calcium hydroxide and magnesium hydroxide precipitates do not dissolve in excess NaOH but aluminium hydroxide does
- Another test could be used to distinguish between the Ca2+ and Mg2+ ions, for example a flame test
- Most transition metals produce hydroxides with distinctive colours
Exam Tip
Be sure to distinguish between the term “colourless” and “clear”. A solution that loses its colour has become colourless. A clear solution is one that you can see through such as water. Solutions can be clear and have colour e.g: dilute copper sulphate.
The document Metal Hydroxides | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches















