Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Introduction to Halides
Introduction to Halides | Chemistry for Grade 10 PDF Download
Silver Nitrate Test
- Acidify the sample with dilute nitric acid (HNO3) followed by the addition of silver nitrate solution, AgNO3
- The acidification is done to remove carbonate ions that might give a false positive result
- If a halide is present it forms a silver halide precipitate:
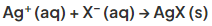
- Depending on the halide present, a different coloured precipitate is formed, allowing for identification of the halide ion
- Silver chloride is white, silver bromide is cream and silver iodide is yellow
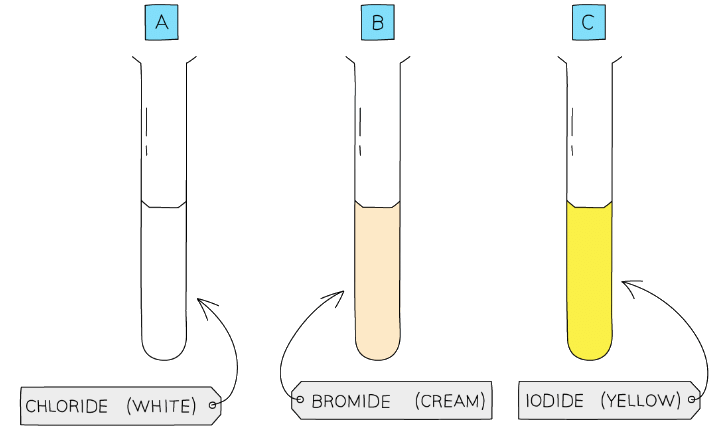 Each silver halide produces a precipitate of a different colour
Each silver halide produces a precipitate of a different colour
Exam Tip
The acidification step in the halide ion test must be done with nitric acid rather than hydrochloric acid, as HCl contains chloride ions which would interfere with the results.
The document Introduction to Halides | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches













