Potable Water & Waste Water Treatment | Chemistry for Grade 10 PDF Download
| Table of contents |

|
| Potable water |

|
| Desalination |

|
| Water for Human Consumption |

|
| Sources of Waste Water |

|
| Required practical |

|
Potable water
Water is essential for life. Water that is safe for humans to drink is called potable water. Potable water is not pure water because it almost always contains dissolved impurities.For water to be potable, it must have sufficiently low levels of dissolved salts and microbes. This is because:
- dissolved salts can sometimes be harmful for humans
- microbes can cause illnesses
Potable water in the UK
- The methods used to make water potable depend on where you live. Starting with fresh water is easier than sea water, as removing the large amount of sodium chloride present in sea water requires a lot of energy.
- In the UK, rain provides enough fresh water to meet the needs of the population. Sometimes during the summer months in some areas of the UK, water reserves run low and people are encouraged to conserve tap water by the use of hosepipe bans.
- Rainwater collects in rivers, lakes and in rocks underground. This water contains low levels of dissolved substances.
Most potable water in the UK is produced from naturally occurring fresh water by:
- passing the water through filter beds to remove insoluble particles
- sterilising the water to kill microbes
The methods used for sterilisation include chlorine, ozone and ultraviolet light.
Desalination
Potable water can be made from sea water, through a process known as desalination. It is preferable to make potable water from fresh water reserves rather than from sea water. This is because removing the large amount of sodium chloride (35 grams in every kilogram of sea water) requires a lot of energy.Desalination can be done by distillation and by reverse osmosis.
Distillation
- Sea water is heated until it boils. The salt remains in the liquid, and the steam is pure water. The steam is cooled and condensed to make potable water.
- Distillation requires a lot of energy to boil the water, and also to cool the steam down to condense it. The waste water is very salty and can be difficult to dispose of in a sustainable way which does not harm marine ecosystems.
Reverse osmosis
Water is put under high pressure and passed through a membrane which has tiny pores (holes) in it. The pores allow water molecules through, but prevent most ions and molecules from passing through. Reverse osmosis requires expensive membranes and also produces a large volume of waste water, so its efficiency is often quite low.Example: Which method of water purification would be best suited to a country in the Middle East which has a large amount of fossil fuel reserves?
Distillation would be the most appropriate method of desalination because the country would be able to use fossil fuels to provide the energy needed at a relatively low cost.
Water for Human Consumption
- Potable water is water that has been processed and is safe for human consumption and daily use
- The difference between pure water and potable water is that pure water is solely made up of H2O molecules, whereas potable water may contain different substances, usually dissolved minerals and salts
- Potable water should have the following characteristics:
- Have a pH between 6.5 and 8.5.
- The dissolved substances (e.g. salts) will be present is very small regulated quantities
- Be free of bacteria or potentially harmful microbes
- Water is considered fresh when it is relatively free from dissolves substances e.g. rainwater
- Water can collect in reservoirs, lakes and rivers and is known as surface water
- In addition, it can collect in aquifers which are porous rocks that store water underground
- This water is called groundwater
Water Treatment
- To obtain potable water, a suitable fresh water source is chosen
- The origin of fresh water depends on the climate in the region in question
- In warmer areas, such as in the south-east of England, water primarily dries up before it can be collected so is found underground
- Despite being relatively low in dissolved substances, there is still a process in place to ensure the fresh water is safe and suitable for use.
- Two important steps in this process are:
- Filtration:
- Debris such as pieces of soil and dirt, small pebbles, twigs, etc. are removed by a wire mesh screen
- After this, other debris is filtered through sand beds and gravel
- Sterilisation:
- Ultraviolet light or ozone can be used to sterilise water or alternatively chlorine gas is bubbled through the water
- This removes any dangerous bacteria or microbes
- Filtration:
- Where aquifers are not present and/or the collection of surface water is limited, the process of desalination must be used to provide potable water to the population
- Desalination involves the treatment of seawater to remove the salt by distillation or reverse osmosis, a process that involves the use of membranes
- When salt water is put through a semi-permeable membrane, only water molecules can pass through it. This happens as the membrane stops larger molecules and ions passing through
- Desalination is an expensive process as it consumes large amounts of energy and is not ideal when producing large quantities of fresh water
- This is used in regions with a very hot climate such as Saudi Arabia
Exam Tip
The way in which potable water is prepared and delivered to a population depends largely on the local conditions of geology.
Sources of Waste Water
- Water is used on a daily basis in a domestic environment. For example, washing-up dishes, showers and baths and cooking
- When you run water down a drain, it passes through sewers and then finally to sewage treatment plants
- Agricultural waste from animal farms and nutrient run-off which is collected from fields produces an abundance of waste water
- Both domestic and agricultural sewage needs to be processed to remove organic matter, harmful microbes, particulates and toxins
- This can then be safely returned to freshwater sources i.e. lakes and rivers
- If this process did not take place, it could potentially pose health risks for the population
- Waste water that is produced by the Haber process and other industrial processes needs to be gathered and treated appropriately
- Harmful chemicals and organic matter are present in industrial waste
- This therefore means that additional treatment has to be in place to ensure it is safe for the environment
Exam Tip
Waste water, sometimes called 'grey water', cannot be allowed to run untreated as it contains dangerous substances and toxins and is detrimental to the environment and health.
Sewage Treatment
- Screening & Grit Removal
- The first stage of treatment removes large materials such as plastic bags and twigs and grit by screening
- Sedimentation
- Sedimentation comes next which occurs in a settlement tank. The water is allowed to stand still in the tank while heavier solids sink to the bottom creating sewage sludge, whilst lighter matter which is also known as effluent, floats to the top
- Aerobic Digestion
- The effluent is removed and treated by biological aerobic digestion
- This involves pumping air into the water to encourage the breakdown of organic matter and other microbes by aerobic bacteria
- Anaerobic Digestion
- Anaerobic digestion is then used to break down the sewage sludge from the bottom of the settlement tank. It is firstly removed and placed in large tanks where bacteria break it down
- Anaerobic digestion releases methane gas as a by product from the organic matter in the sludge. Methane gas is used as a source of energy and the leftover, digested waste as a fertiliser
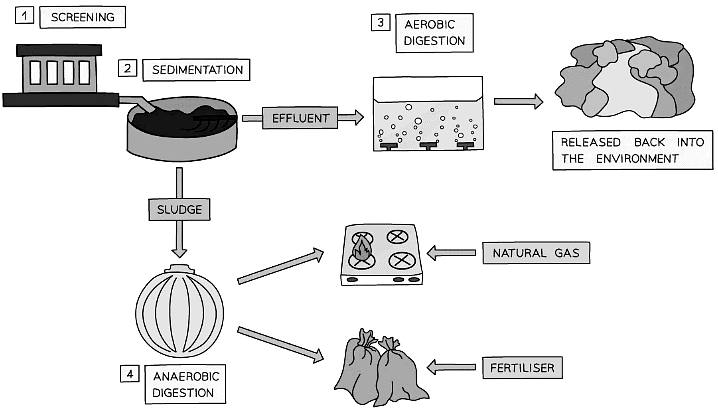 Diagram showing the stages of waste water treatment
Diagram showing the stages of waste water treatment
- When there are toxic substances within waste water, it is important to use additional phases of treatment
- This can include using membranes, adding additional chemicals e.g. to precipitate metals out of solution, and also U.V. radiation
- Sewage water is often treated in areas where there is little freshwater available
- Though this process is longer than processing and preparing freshwater, it uses less energy than the desalination of salt water
Exam Tip
Waste water comes from many different sources so the process of waste water treatment is complex and involves various stages.
Required practical
Analysis and purification of water
It is important in this core practical to use apparatus and substances carefully and safely, and to observe chemical changes.
Eye protection must be worn at all times.
Aims
- To analyse samples of water from different sources in terms of pH and the presence of dissolved solids.
- To distil sea water to obtain potable water.
Method - Part 1 (Analysing water samples)
- For each sample of water you are given, test the pH using either a pH meter or universal indicator and an appropriate colour chart. Record your observations carefully.
- For each sample of water, pour 50 cm3 into a clean pre-weighed evaporating basin. Heat gently over a Bunsen burner, tripod and gauze until no liquid remains. Allow to cool, then weigh the evaporating basin again and calculate the mass of solid that remains in the evaporating basin.
Analysis - Part 1 (Analysing water samples)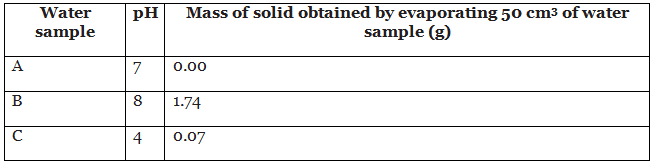
Example 1: Which sample was sea water?
Sample B was sea water. It contained the highest mass of dissolved solids.
Example 2: Which sample was obtained from a region affected by acid rain?
Sample C was acidic, so it could have been obtained from a region affected by acid rain.
Method - Part 2 (Distillation)
- Set up your distillation apparatus as demonstrated by your teacher. You may be able to use a conventional condenser like this:
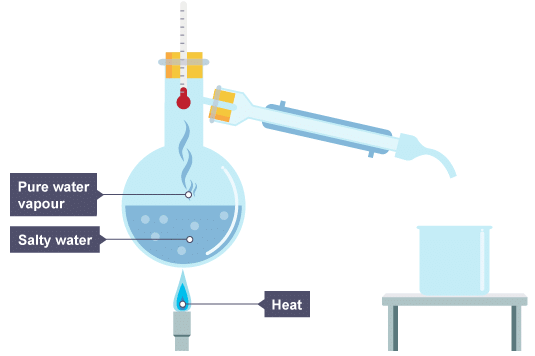 Salty water is heatedOr you might be using apparatus like this:
Salty water is heatedOr you might be using apparatus like this: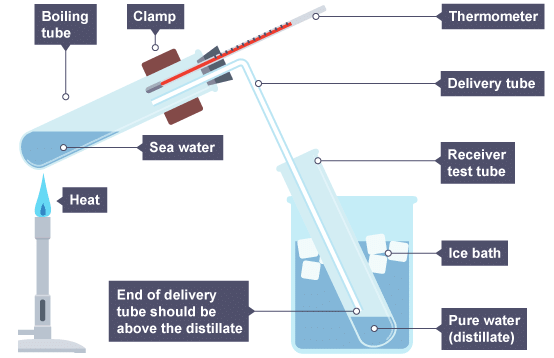
- Heat your sea water solution until it boils gently.
- After a period of time you should see distilled water being produced.
Example 1: Why is it not safe to drink the distilled water sample?
It might be contaminated because the apparatus might not have been cleaned properly before the practical.
Example 2: Why was it important to test heat 50 cm3 of each water sample to dryness in the first part of the practical?
So the mass of the dissolved solids could be compared in a valid test.
Example 3: How could you test to see if the distilled water contained sodium ions and chloride ions?
Sodium ions would give a yellow colour in a flame test. Chloride ions would give a white precipitate with silver nitrate solution after a small amount of nitric acid was added. For a reminder about these chemical tests, see this guide on chemical tests.
Example 4: It is essential that the end of the delivery tube in the method described by the second diagram above is above the level of the filtrate. This is for safety reasons, when you stop heating the sea water mixture. Explain what would happen if the heat was removed and the end of the delivery tube was below the surface of the distillate.
As the gas inside the boiling tube and delivery tube cools, it will contract, sucking the distillate up the delivery tube. When this cool water reaches the hot glass, it may cause the glass to break.
Hazards, risks and precautions
Evaluate the hazards and the precautions needed to reduce the risk of harm. For example: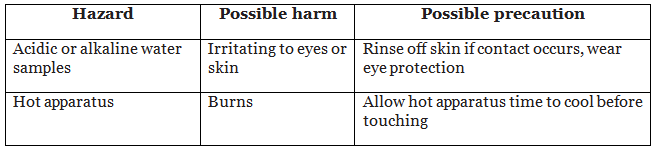
|
73 videos|87 docs|21 tests
|



















