Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Corrosion & Its Prevention
Corrosion & Its Prevention | Chemistry for Grade 10 PDF Download
| Table of contents |

|
| Degradation of Metals in the Environment |

|
| Investigating Conditions for Rusting |

|
| Rust Prevention Methods |

|
| Sacrificial corrosion |

|
Degradation of Metals in the Environment
- Most metals react with substances when exposed to the environment for prolonged periods
- This causes degradation of the metal in a process called corrosion
- Corrosion occurs at the surface of the metal only
- Rusting is a chemical reaction between iron, water and oxygen to form the compound iron(III) oxide or hydrated iron(III) oxide
- Oxygen and water must be present for rusting to occur
- Rusting is a redox process and it occurs faster in salty water since the presence of sodium chloride catalyses the process
- The equation for the rusting of iron is:
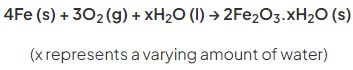
- Rust is a soft solid substance that flakes off the surface of iron easily, exposing fresh iron below which then undergoes rusting
- This means that over time all of the iron rusts and its structure becomes weakened
- This is a major concern as iron is used extensively in industries such as transport and construction
- Aluminium is another metal that undergoes corrosion but in a slightly different way to iron
- Aluminium reacts with oxygen to produce aluminium oxide, Al2O3
- The aluminium oxide forms a tough protective layer that covers the aluminium, preventing further corrosion
Exam Tip
Corrosion and rusting are not the same process. Corrosion is the general term used to describe the degradation of metal surfaces whereas rusting is the specific type of corrosion that happens to iron.
Investigating Conditions for Rusting
- Oxygen and water must be present for rust to occur
- You can investigate the conditions needed for rusting by setting up a series of control test tubes as shown below
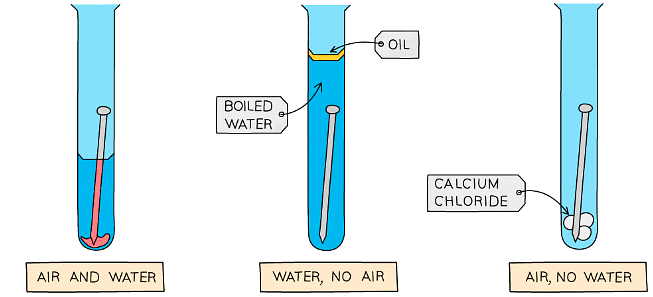 Diagram showing how the conditions for rusting can be investigated
Diagram showing how the conditions for rusting can be investigated
Method:
- Set up the apparatus as shown in the diagram
- The water in the second test tube is boiled to remove any dissolved oxygen
- The oil provides a barrier to prevent oxygen diffusing into the boiled water
- Calcium chloride is a drying agent in the third test tube
- Leave the apparatus for a few days to give it time to react
Results:
- The nail on the left rusts as it is in contact with both air (which contains oxygen) and water
- The nail in the middle does not rust as it is not in contact with air
- The nail on the right does not rust as it is not in contact with water (calcium chloride absorbs any water molecules present due to moisture)
- The results show that both air and water must be present for rusting to occur
Rust Prevention Methods
Barrier Methods
- Rust can be prevented by coating iron with barriers that prevent the iron from coming into contact with water and oxygen
- However, if the coatings are washed away or scratched, the iron is once again exposed to water and oxygen and will rust
- Unlike some other metals, once iron begins to rust it will continue to corrode internally as rust is porous and allows both air and water to come into contact with fresh metal underneath any barrier surfaces that have been broken or scratched
- Common barrier methods include: paint, oil, grease and plastic
Galvanising / Sacrificial protection
- Iron can be prevented from rusting making use of metals higher in reactivity than iron
- Galvanising is a process where the iron to be protected is coated with a layer of zinc
- ZnCO3 is formed when zinc reacts with oxygen and carbon dioxide in the air and protects the iron by the barrier method
- If the coating is damaged or scratched, the iron is still protected from rusting because zinc preferentially corrodes as it is higher up the reactivity series than iron
- Compared to iron it loses its electrons more readily:
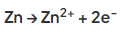
- The iron stays protected as it accepts the electrons released by zinc, remaining in the reduced state and thus it does not undergo oxidation
- The electrons donated by the zinc react with hydrogen ions in the water producing hydrogen gas:
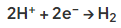
- Zinc therefore reacts with oxygen and water and corrodes instead of the iron
Sacrificial corrosion
- Sacrificial corrosion occurs when a more reactive metal is intentionally allowed to corrode
- An example of this occurs with ships' hulls which sometimes have large blocks of magnesium or magnesium alloys attached
- The blocks slowly corrode and provide protection to the hull in the same way the zinc does by pushing electrons onto the iron which prevents it from being reduced to iron(III) ions
The document Corrosion & Its Prevention | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches














