Physical and Chemical Changes Class 7 Notes Science Chapter 5
| Table of contents |

|
| Chemical Substances |

|
| Chemical Symbols |

|
| Chemical Formulae |

|
| Chemicals and Chemical Changes |

|
| Physical Changes |

|
| Key Words |

|
| Activity 1: |

|
| Activity 2: |

|
Chemical Substances

Chemical substances are made of atoms and molecules. Atoms may or may not exist independently. Molecules can exist independently
Elements
Substance made up of only one kind of atoms. Cannot be broken down into simpler substances by chemical means.
Examples:- Silver, Gold, Aluminium, Hydrogen, Oxygen
Compounds
Substance formed when two or more elements combine chemically. Smallest particle of a compound is a molecule which shows all the properties of that compound.
Examples:- Water (hydrogen + oxygen), Carbon dioxide (carbon + oxygen)
Mixtures

Two or more substances simply mixed together, but not chemically combined. Retains the properties of its components.
Examples:- Air (nitrogen, oxygen, carbon dioxide, water vapour, argon, and particles of dust, smoke, and many others), Iron filings and sulphur mixture (iron is still attracted by a magnet and sulphur remains as a powder)
Chemical Symbols
Chemical symbols are shorthand notations used to represent the elements in the periodic table. They consist of one or two letters, usually the first letter of the element's name. They are used in chemical equations, formulas, and reactions.
Understanding Chemical Symbols:
Each element has its own unique chemical symbol. The first letter of the symbol is always capitalized, and the second letter (if present) is always lowercase. Some symbols are derived from the element's Latin name, while others are based on their common name or origin.
Examples of Chemical Symbols
- Hydrogen: H
- Carbon: C
- Oxygen: O
- Nitrogen: N
- Sodium: Na
- Chlorine: Cl
- Gold: Au
- Silver: Ag
Using Chemical Symbols
Chemical symbols are used in chemical equations to represent the reactants and products. They are also used in formulas to indicate the composition of compounds and molecules. Chemical symbols are used in lab reports, scientific papers, and textbooks.
Atomicity
Atomicity refers to the number of atoms present in a molecule. The chemical symbol of an element is used to represent one atom of that element. For example, the symbol 'H' represents one atom of hydrogen, while 'O' represents one atom of oxygen.
Isotopes
Isotopes are atoms of the same element that have different numbers of neutrons in their nuclei. Isotopes are identified by adding the mass number to the chemical symbol. For example, carbon-12 is represented as 'C-12', while carbon-14 is represented as 'C-14'.
Chemical Formulae
A chemical formula represents a chemical compound using chemical symbols of its constituent elements. The chemical formula can be derived by knowing the constituent elements and their valency. Examples of chemical formulae of some compounds: Water: H2O, Carbon dioxide: CO2
Valency
The combining capacity of an element's atom is called valency. The valency of some common elements are: Hydrogen: 1, Oxygen: 2, Carbon: 4
Writing a Chemical Formula
To write a chemical formula, you need to know the symbols and valencies of the elements. The valency is the number of bonds an atom can form with other atoms. The valency of an element is usually indicated by a positive or negative sign. The formula for a compound is written by balancing the valencies of the elements. For example, the formula for sodium chloride (table salt) is NaCl, which means one sodium atom and one chlorine atom are combined
Examples of writing chemical formulae using the steps above:
- Water: H2O (hydrogen valency: 1, oxygen valency: 2)
- Carbon dioxide: CO2 (carbon valency: 4, oxygen valency: 2)
Chemical Equations
Chemical equations are a representation of a chemical reaction using symbols and formulas. Chemical equations help us understand how different substances react with each other and what products are formed.
Parts of a Chemical Equation
- Reactants: The substances that are present at the beginning of a chemical reaction.
- Products: The substances that are formed as a result of the chemical reaction.
- Arrow: Indicates the direction in which the reaction is proceeding.
Steps to write a chemical equation:
- Identify the reactants and products of the reaction
- Write the names of the reactants on the left-hand side with a '+' sign between them and write the names of the products on the right-hand side with a '+' sign between them. Place an arrow () between the two sides, with the arrow-head pointing towards the products, i.e., between reactants and products. This equation is called a word equation.
- Replace the names of the products and the reactants with symbols and formulae. This gives the chemical equation.
- Balance the equation to ensure that the number of atoms of each element on both sides is equal.
Balancing an equation:
- Count the number of atoms of each element on both sides of the equation.
- Balance the equation by adjusting the coefficients of the reactants and/or products to ensure that the number of atoms of each element is equal on both sides.
Writing Chemical Equations for Given Reactions
- Reaction 1: magnesium + oxygen → magnesium oxide
- Word equation: Magnesium + oxygen → Magnesium oxide
- Chemical equation: 2Mg + O2 → 2MgO
- Reaction 2: sodium + chlorine → sodium chloride
- Word equation: Sodium + chlorine → Sodium chloride
- Chemical equation: 2Na + Cl2 → 2NaCl
- Reaction 3: aluminium + chlorine → aluminium chloride
- Word equation: Aluminium + chlorine → Aluminium chloride
- Chemical equation: 2Al + 3Cl2 → 2AlCl3
Uses of Chemical Equations
- Chemical equations are used in industries to make different products.
- They are used in agriculture to create fertilizers and pesticides.
- They are also used in medicine to create different drugs.
Chemicals and Chemical Changes

Chemical changes involve the creation of new substances with different properties from the original substances. Examples of chemical changes include rusting of iron and browning of vegetable and fruit surfaces.
- Rusting of Iron:
Iron rusts when it reacts with oxygen in the presence of water. Rust is a type of compound known as iron oxide. Rusting can be prevented by painting or coating iron surfaces. - Browning of Vegetable and Fruit Surfaces:
Browning is a chemical change that occurs in fruits and vegetables when they are cut or bruised. This process involves the oxidation of enzymes and chemicals in the plant tissues. Browning can be slowed down by adding lemon juice or storing the produce in the refrigerator. - Reaction Between Vinegar and Baking Soda:
When vinegar and baking soda are mixed, a chemical reaction occurs that produces carbon dioxide gas. This reaction is an example of an acid-base reaction. The gas produced can be used to inflate a balloon or create a homemade volcano. - Reaction Between Copper Sulphate Solution and Iron Nails:
When iron nails are placed in a copper sulphate solution, a chemical reaction occurs that produces iron sulphate and copper. This reaction is an example of a displacement reaction. The iron nails become coated in copper and the blue colour of the copper sulphate solution fades.
Physical Changes
Physical changes refer to the changes in the state, size, shape, or texture of a substance that do not involve any change in its chemical composition.
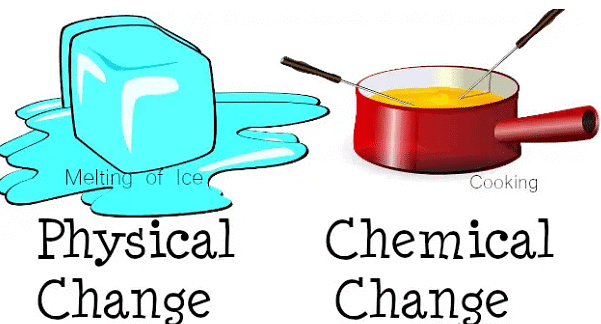 Types of Physical Changes:
Types of Physical Changes:- Change in State: A substance can change its state from solid to liquid or from liquid to gas by changing its temperature or pressure.
- Change in Size: A substance can undergo a change in size by expanding or contracting due to a change in temperature or pressure.
- Change in Shape: A substance can change its shape by deformation due to an external force.
- Change in Texture: A substance can change its texture due to an external force, such as grinding or cutting.
Crystallization
Crystallization is a physical change that involves the formation of crystals from a solution or a melt. The process involves the slow cooling of a substance to allow the molecules to arrange themselves in a regular pattern, forming a crystal.
Applications of Physical Changes:
- Cooking: Physical changes are used in cooking to change the texture and appearance of food.
- Cleaning: Physical changes are used in cleaning to remove stains and dirt from surfaces.
- Manufacturing: Physical changes are used in manufacturing to change the size, shape, and texture of materials.
Key Words

Activity 1:
Aim: To observe a chemical change
Material needed: Vinegar, baking soda, beaker, spoon
Method:
Take a beaker and add some vinegar to it. Add a spoonful of baking soda to the vinegar. Observe what happens.
Observation: The vinegar and baking soda react to form bubbles of carbon dioxide gas.
Conclusion: This is a chemical change because the reaction forms a new substance with different properties from the original substances.
Conclusion: Chemicals are all around us, and chemical changes occur when two or more substances react to form new substances. Writing chemical formulas and equations helps us understand these changes. Physical changes occur when the form or appearance of a substance changes, but the substance remains the same. Crystallization is a process in which a solid forms from a solution.
Activity 2:
Aim: To observe a chemical change.
Materials Needed:
- Vinegar
- Baking soda
- Glass beaker
- Balloon
Method:
- Add a small amount of baking soda to a glass beaker.
- Add vinegar to the beaker.
- Quickly cover the beaker with a balloon.
- Observe what happens.
Observation:
- The mixture will start to bubble and fizz.
- The balloon will start to inflate.
Conclusion:
The reaction between baking soda and vinegar produces carbon dioxide gas, which inflates the balloon. This is an example of a chemical change, where two substances react to form a new substance.
|
139 videos|151 docs|18 tests
|















