Worksheets With Solutions: Chemical and Chemical Changes Class 7 Worksheet Science Chapter 5
| Table of contents |

|
| Section A: Multiple Choice Questions |

|
| Section B: Short Questions |

|
| Section C: Fill in the Blanks |

|
| Section D: Match the Column |

|
| Section E: True or False |

|
Section A: Multiple Choice Questions
Q.1. Which of the following is not a chemical substance?
(a) Oxygen
(b) Water
(c) Sugar
(d) Sand
Correct Answer is Option (d)
Sand is not a pure substance as it is made up of various minerals and rocks. Chemical substances are pure substances that have a defined chemical composition and properties.
Q.2. Which of the following is a chemical change?
(a) Melting of ice
(b) Burning of wood
(c) Cutting of paper
(d) Mixing of salt and water
Correct Answer is Option (d)
Burning of wood is a chemical change as it involves a chemical reaction between the wood and oxygen in the air, resulting in the formation of new substances.
A chemical change involves the formation of new substances with different chemical properties. Burning of wood involves the breakdown of complex organic molecules present in wood into simpler substances, such as carbon dioxide and water vapor.
Q.3. What is the chemical symbol for gold?
(a) Ag
(b) Au
(c) Fe
(d) Pb
Correct Answer is Option (b)
Chemical symbols are shorthand notations used to represent elements. Each element has a unique chemical symbol, which is usually derived from its name in English or Latin.
Q.4. Which of the following is a physical change?
(a) Rusting of iron
(b) Baking of a cake
(c) Digestion of food
(d) Burning of fuel
Correct Answer is Option (a)
A physical change involves a change in the physical properties of a substance without the formation of new substances. Rusting of iron involves the formation of a reddish-brown oxide layer on the surface of iron due to its reaction with oxygen and moisture in the air.
Q.5. What is the chemical formula for water?
(a) H2SO4
(b) CO2
(c) NaCl
(d) H2O
Correct Answer is Option (d)
A chemical formula is a shorthand notation used to represent a compound. It shows the types of atoms present in the compound and their relative numbers.
Section B: Short Questions
Q.1. Define a chemical change.
A chemical change is a process in which one or more substances are transformed into new substances with different chemical properties. It involves the breaking and forming of chemical bonds between atoms or molecules. Examples of chemical changes include burning of wood, rusting of iron, and digestion of food.
Q.2. What is the difference between a chemical substance and a mixture?
A chemical substance is a pure substance that has a defined chemical composition and properties. It consists of one or more types of atoms or molecules that are chemically bonded together. Examples of chemical substances include water, sodium chloride, and oxygen gas. A mixture, on the other hand, is a combination of two or more substances that are physically mixed together but not chemically bonded. Examples of mixtures include air, sand, and sugar water.
Q.3. Write the chemical formula for sodium chloride.
The chemical formula for sodium chloride is NaCl. It represents a compound that is made up of one sodium atom and one chlorine atom, which are held together by an ionic bond.
Q.4. Give an example of a physical change.
An example of a physical change is the melting of ice. It involves a change in the physical state of water from solid to liquid without the formation of new substances. The melting point of ice is 0°C, and when heat is applied, the ice absorbs energy and breaks its hydrogen bonds, resulting in the formation of liquid water.
Q.5. Explain what happens during crystallization.
Crystallization is a process in which a solid substance is transformed into a crystal with a regular, repeating pattern of atoms or molecules. It involves the cooling of a hot, saturated solution, which causes the solute molecules to come together and form a crystal lattice. An example of a substance that can be crystallized is sugar, which is often purified through the process of crystallization.
Section C: Fill in the Blanks
Q.1. Chemical substances are made up of _____________.
Chemical substances are made up of Atoms
Q.2. The shorthand way of representing an element is its _______________.
The shorthand way of representing an element is its Chemical symbol
Q.3. The combination of symbols and numbers which indicates the number of atoms in a molecule is called the _______________.
The combination of symbols and numbers which indicates the number of atoms in a molecule is called the Chemical formula.
Q.4. A chemical reaction is represented by a _____________.
A chemical reaction is represented by a Chemical equation.
Q.5. The process of separating a solid from a solution by cooling it is called _______________.
The process of separating a solid from a solution by cooling it is called Crystallization.
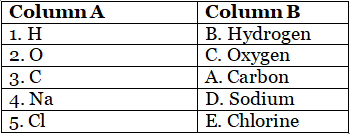
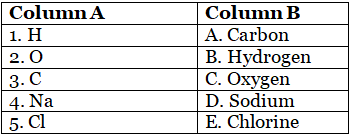
Section D: Match the Column
Match the following symbols with their respective elements:
Section E: True or False
Q.1. A chemical change is reversible.
False
Q.2. Physical changes do not change the nature of the substance.
True
Q.3. Chemical formulae are used to represent the composition of a substance.
True
Q.4. Crystallization is a physical change.
False
Q.5. Chemical reactions occur when substances are heated.
False
|
139 videos|151 docs|18 tests
|