Acids, Bases and Salts Class 7 Notes Science Chapter 2
| Table of contents |

|
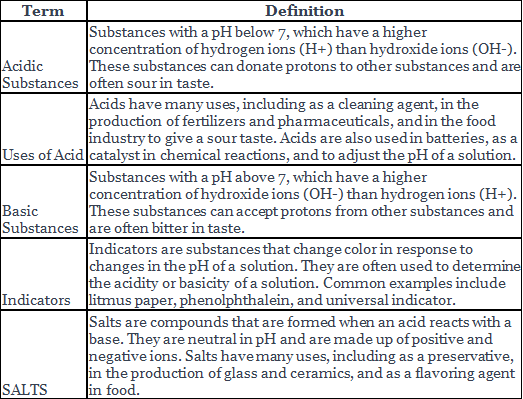
| Acidic Substances |

|
| Indicators |

|
| SALTS |

|
| Key Words |

|
| Activity |

|
Ms Rai, the science teacher, explains about different types of substances to her class Sour and bitter tastes are due to the presence of acid and base respectively. Let's learn more about these substances.
Acidic Substances
- Mineral Acids
Mineral acids, such as hydrochloric acid, sulphuric acid, and nitric acid, are produced from minerals found in nature and are highly reactive. - Organic acids
Organic acids occur naturally in animal and plant materials. Examples of organic acids with their sources are given in a table. - Strong and Weak Acids
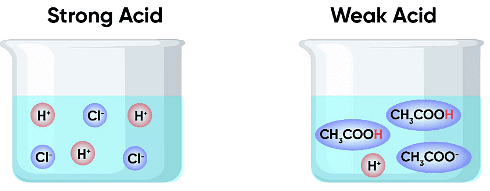
Strong and Weak Acids Strong acids, such as mineral acids, are highly corrosive and can cause severe burns Weak acids, mostly organic acids, are not as destructive as strong acids Acetic acid, which is used to make vinegar, is an example of weak acid Carbonic acid, found in fizzy drinks and soda water, is also a weak acid but not an organic acid. - Properties of Acids
Acids are sour to taste and corrosive in nature. They can corrode metals such as iron and aluminium. Mineral acids can burn human tissues and damage clothes and paper. Acids are generally stored in glassware to prevent reactions with other materials. Bases are slippery and bitter in taste. Soap is an example of a base Bases can be classified as strong or weak, just like acids. - Properties of Bases
Bases are bitter in taste and slippery in nature. They can corrode materials such as cloth and paper. Strong bases are highly reactive and can cause burns on contact with skin. - Neutralization
When an acid and a base react, they cancel each other's properties and form a neutral substance, such as water and salt
This reaction is called neutralization and is used in various applications, such as in the production of fertilizers and medicines - Salts
Salts are formed by the reaction of an acid and a base.
They are neutral in nature and have various uses, such as in food preservation and water softening.
Properties of Acids:
An acid is a chemical compound that has a pH less than 7. Some common properties of acids are:
- Acids are soluble in water.
- Acids have a sour taste.
- Acids react with metals to produce hydrogen gas.
- Acids turn blue litmus paper red.
Uses of Acids
- Hydrochloric acid is used to remove deposits from boilers and to clean sinks and sanitary ware.
- Sulphuric acid is used in car batteries, paints, drugs, dyes, and fertilizers.
- Nitric acid is used by goldsmiths for cleaning gold and silver ornaments and in the production of fertilizers.
- Acetic acid is used in vinegar to enhance the flavor of food and as a cleansing agent in products meant for cleaning windows, floors, utensils, etc.
Types of Acids
Acids can be classified as dilute or concentrated based on the amount of water in them. Some common acids are:
- Hydrochloric acid (HCI)
- Sulphuric acid (H2SO4)
- Nitric acid (HNO3)
- Acetic acid (CH3COOH)
Properties of Bases
A base is a chemical compound that has a pH more than 7. Some common properties of bases are:
- Bases are soluble in water.
- Bases have a bitter taste.
- Bases turn red litmus paper blue.
- Bases feel slippery to the touch.
Uses of Bases
Bases also have various uses in our daily lives. Some common uses of bases are:
- Sodium hydroxide (NaOH) is used to make soap, paper, and textiles.
- Calcium hydroxide (Ca(OH)2) is used in cement and in agriculture to neutralize acidic soil.
- Ammonium hydroxide (NH4OH) is used in cleaning products like window cleaners, floor cleaners, and bathroom cleaners.
Properties of Salts
Salts are chemical compounds made up of a metal and a non-metal. Some common properties of salts are:
- Salts are usually soluble in water.
- Salts have a neutral pH.
- Salts can conduct electricity when dissolved in water.
Uses of Salts
Salts also have various uses in our daily lives. Some common uses of salts are:
- Sodium chloride (NaCl) is used in food as a flavor enhancer and as a preservative.
- Calcium carbonate (CaCO3) is used in toothpaste, antacids, and as a dietary supplement.
- Magnesium sulphate (MgSO4) is used in fertilizers and in medicine as a laxative.
Basic Substances
Substances containing a base are called basic substances. Some examples of basic substances are sodium hydroxide and calcium hydroxide. Bases may have a strong irritating odour and should be used with caution as they can harm the skin and eyes.
Strong and Weak Bases
Like acids, bases can be strong or weak. Strong bases are very corrosive and can burn the skin, and should be handled carefully. Caustic soda or sodium hydroxide (NaOH) and caustic potash or potassium hydroxide (KOH) are strong and corrosive bases. Copper hydroxide, zinc hydroxide, and ammonium hydroxide are weak bases.
Properties of Bases
Some of the characteristic properties of bases are:
Bases are bitter to taste.
Solutions of bases are slippery to touch. Bases may or may not be soluble in water. Bases that can dissolve in water are called alkalis.
Uses of Bases
Some of the common uses of bases are:
Calcium Hydroxide (Slaked lime) [Ca(OH)2] is used to neutralize the acidity in soils, an ingredient in whitewash and mortar, a component of the Bordeaux mixture used for protecting agricultural crops from pests, and in the preparation of dry mixes for painting and decorating.
Magnesium Hydroxide (Milk of magnesia) [Mg(OH)2] is used as an antacid or a laxative. It helps to correct excess acidity in the stomach.
Sodium Hydroxide (Caustic soda) (NaOH) is used in the manufacture of paper and textiles, occasionally used to unblock drains in homes, and in the manufacture of soaps and detergents.
Indicators
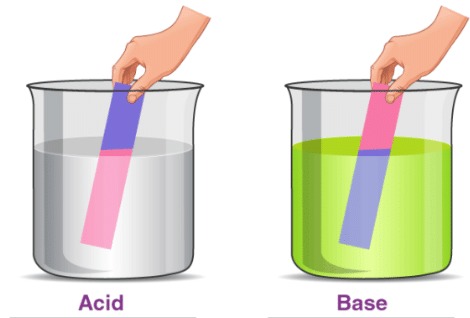 Indicators are chemicals that change colour when they come in contact with acids and bases. Litmus is a common indicator used in laboratories that changes colour depending on whether the compound is an acid or a base. Blue litmus paper turns red under acidic conditions, and red litmus paper turns blue under basic conditions. Phenolphthalein and methyl orange are also used as indicators to detect the presence of acids and bases.
Indicators are chemicals that change colour when they come in contact with acids and bases. Litmus is a common indicator used in laboratories that changes colour depending on whether the compound is an acid or a base. Blue litmus paper turns red under acidic conditions, and red litmus paper turns blue under basic conditions. Phenolphthalein and methyl orange are also used as indicators to detect the presence of acids and bases.
Universal Indicators
Scientists use pH numbers to measure the strength of an acid or a base. The pH scale ranges from 1 to 14, where pH 1 is strongly acidic, pH 14 is strongly basic, and pH 7 is neutral. A universal indicator is a mixture of different indicators that gives a different colour for different pH values. pH paper can be soaked in this indicator and used to measure the pH of a solution.
Natural Indicators
Indicators can also be prepared from brightly coloured parts of plants, such as flowers, roots, stems, and leaves. Indicator solutions can be prepared by boiling coloured parts of the plant in water and straining out the plant part. Red cabbage juice is a common natural indicator that changes colour to deep red with acids, purple with neutrals, and green and yellow with bases. Turmeric is another natural indicator that reacts with bases to form a red compound.
SALTS
Salts are substances formed by the reaction between an acid and a base. This reaction is called a neutralization reaction.
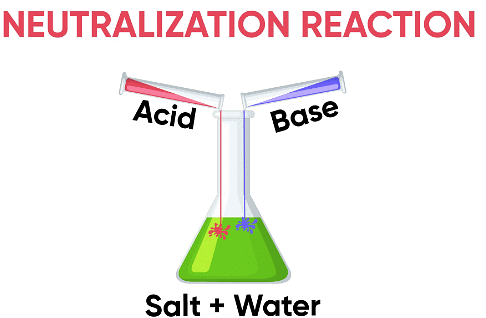 Example: reaction between vinegar and baking soda.
Example: reaction between vinegar and baking soda.Formation of Salt
Vinegar contains an acid, and baking soda contains a base. When they react, salt is formed along with water and carbon dioxide gas.
Table Salt Formation
Common table salt is formed by the reaction between hydrochloric acid (HCI) and sodium hydroxide (NaOH).
Types of Salts
Salts can be acidic, basic, or neutral. Acidic salts are formed when a strong acid reacts with a weak base. Basic salts are formed when a strong base reacts with a weak acid. Neutral salts are formed when a strong acid reacts with a strong base.
Properties of Salts
- Salts have high melting and boiling points.
- They are generally soluble in water, but some salts are insoluble.
- Salts can have water of crystallization, which gives them their shape and color.
- Heating hydrated salts causes them to lose their water of crystallization and become anhydrous.
Uses of Salts
Salts are used for various purposes, such as cooking, preserving food, and making medicines. Table salt is used in cooking, and Epsom salt is used for treating sore muscles.
Key Words
Activity
Aim: To distinguish between an acidic and basic substance using an indicator.
Materials needed: Litmus paper, lemon juice, baking soda, water, and two test tubes.
Method: Take two test tubes and fill one with lemon juice and the other with baking soda solution. Dip a red litmus paper in the lemon juice and observe the color change. Then, dip a blue litmus paper in the baking soda solution and observe the color change.
Observation: The red litmus paper turns blue in the baking soda solution, indicating that it is a basic substance. The blue litmus paper turns red in the lemon juice, indicating that it is an acidic substance.
Conclusion: Based on the observations, we can conclude that the baking soda solution is a basic substance, and the lemon juice is an acidic substance.
Conclusion: Acids, bases, and salts are essential substances that we come across in our daily lives. Understanding their properties and uses is important for various reasons. This chapter gives a basic idea about these substances and their applications, which will help in further studies.
|
139 videos|151 docs|18 tests
|