Temperature and Heat Class 7 Notes Science
| Table of contents |

|
| Introduction |

|
| Temperature |

|
| Modes of Heat Transfer |

|
| Key Words |

|
| Activity: |

|
Introduction
Temperature and heat are two important concepts related to hotness and coldness that we experience in our daily lives. We feel hot or cold based on the temperature of the surrounding environment and the objects we come in contact with. This chapter will help us understand the meaning of temperature, how it is measured, and how it relates to the concept of heat.
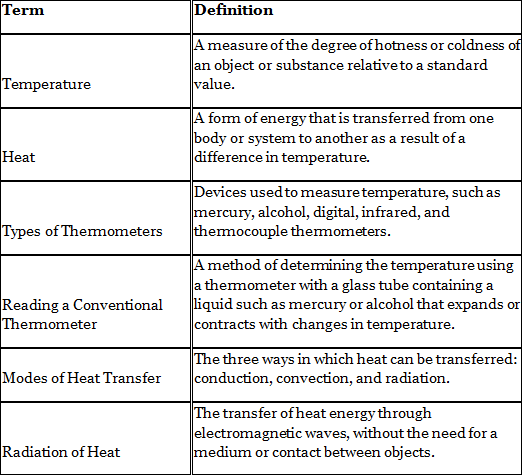
Temperature
The degree of hotness or coldness of a body or a place is called temperature. It is measured using an instrument called a thermometer. Our sense of touch can be misleading when it comes to determining the temperature of an object or a place.An experiment can be conducted to demonstrate this fact.
"Activity
The experiment requires three glasses of water - hot, cold, and room temperature.
By dipping one hand's index finger in hot water and the other in cold water and then placing them in the room temperature water, we can observe that both fingers give different readings.
The finger that was in hot water will feel the room temperature water as cold, while the finger that was in cold water will feel it as hot.
This is because our sense of hotness and coldness is relative to our prior experience."
Heat
- Heat is a form of energy that flows between two objects due to a temperature difference
- Heat flows from a hotter object to a colder object until both reach the same temperature
- Conductors and insulators affect the flow of heat
Temperature Scales
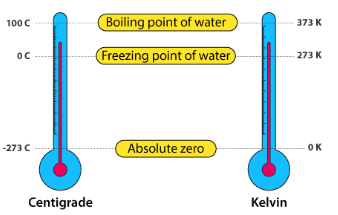
Different units are used to measure temperature, represented by different temperature scales. Two commonly used scales are the Celsius and Fahrenheit scales, and there is a third scale, the Kelvin scale. A temperature scale is defined by choosing two reference temperatures and dividing the difference between them into a certain number of divisions.
Celsius Scale
The Celsius scale is indicated by "C" and is divided into 100 degrees. The melting point of pure ice is taken as 0°C and the boiling point of pure water as 100°C.
Fahrenheit Scale
The Fahrenheit scale is indicated by "F" and is divided into 180 degrees. The melting point of pure ice is taken as 32°F and the boiling point of pure water as 212°F.
Conversion Formula
Two simple equations can be used to convert one temperature scale into another: F = (C x 9/5) + 32 and C = 5(F - 32)/9.
Example 1: Convert 86°F to °C using the formula C = 5(F - 32)/9, the result is 30°C.
Example 2: To convert 45°C to °F using the formula F = (C x 9/5) + 32, the result is 113°F.
Types of Thermometers
There are different types of thermometers: mercury thermometer, alcohol thermometer, and digital thermometer. Each type of thermometer has its own advantages and disadvantages.
- Mercury Thermometer
In 1714, Gabriel Fahrenheit invented the first mercury thermometer. Mercury is used in thermometers because it remains in the liquid state for a wide range of temperatures. It is easy to see because of its silvery grey colour and does not stick to glass. Mercury has a fairly uniform rate of expansion for a wide range of temperatures. - Alcohol Thermometer
Thermometers using alcohol have some advantages over mercury thermometers. Alcohol is cheaper and less harmful than mercury. Alcohol thermometers can measure much lower temperatures than mercury. However, they cannot measure temperatures higher than 78°C. - Digital Thermometer
Digital thermometers do not use mercury and contain a 'thermistor'. The current passing through a thermistor depends on its temperature. Another small device inside a digital thermometer measures the current passing through the thermistor and displays the temperature on an LCD. - Uses of Thermometers
Thermometers are used to measure body temperature, air temperature, water temperature, etc. They are used in various fields such as medicine, cooking, meteorology, and science.
Reading a Conventional Thermometer
Types of thermometer
(a) Laboratory thermometer
(b) Clinical thermometer/Mercury thermometer
(c) Digital thermometer
A laboratory thermometer. It consists of a thin glass tube which is sealed at one end and has a bulb at the other end. The bulb is generally filled with mercury or alcohol, depending on whether it is a mercury or an alcohol laboratory thermometer. Mercury appears as a silvery grey line and alcohol looks like a red line in the thermometer. To read the temperature on this thermometer, you just read the number on the scale at the tip of the silvery-grey or red line. Remember to mention the unit used (Celsius or Fahrenheit). The range of temperatures which a laboratory thermometer can measure is from -10°C to 110°C. A clinical thermometer is generally a mercury thermometer though digital thermometers are becoming quite popular.
There are two characteristic features of a mercury clinical thermometer
- There is a little arrow (at 98.4 or 98.6°F) showing the normal body temperature.
- There is a constriction or 'kink' in the tube near the bulb. This kink has been made to ensure that the mercury in the thermometer does not contract (and flow back into the bulb) before the temperature has been read.
To read the body temperature of any person with a clinical thermometer, follow the steps given below
- First shake the thermometer a few times so that the mercury (the silvery grey line) goes below the 'normal' (body temperature) mark.
- Place the thermometer in the arm pit or under the tongue of the person whose temperature is to be taken. Wait for two minutes.
- Take out the thermometer and read the temperature at which the silver line (of mercury) ends. Keep the thermometer at your eye level for an accurate reading.
Units of Temperature
The unit of temperature used in the International System of Units (SI) is the Kelvin (K). Celsius (°C) and Fahrenheit (°F) are also commonly used units of temperature.
Heat and Energy
Heat is a form of energy that flows from hotter objects to cooler objects. The unit of heat energy is the joule (J). Heat energy can be transferred through three modes: conduction, convection, and radiation.
Effects of Heat
Heat can cause changes in the state of matter, such as melting, boiling, and evaporation. Heat can also cause expansion and contraction of materials. Extreme heat can be harmful to living organisms and can lead to heat exhaustion or heat stroke.
Heat - A Form of Energy
Heat energy is a type of energy that flows between two bodies when there is a difference in temperature. When heat energy flows into a body, it warms the body, and when it flows out of the body, it cools the body. Heat flow always occurs from a region of higher temperature to a region of lower temperature. Heat flow continues until the temperatures of both the hot and the cold body become the same.
- Activity to Demonstrate Heat Flow
The aim of the activity is to show that heat energy flows from a hot body to a cold body. Materials needed include a coin, tongs, boiling water, cold water, and two glasses. Boil water in a vessel and drop a coin in the boiling water. After the coin gets really hot, use tongs to pick up the hot coin and drop it in one of the glasses filled with water. After about 2 minutes, dip your finger in the two glasses, first in the glass without the coin and then in the glass with the coin. The water in the glass in which the hot coin was dropped will be warmer, indicating that heat energy has been transferred from the hot body (coin) to the cold body (water). - Measuring Heat
Heat energy is measured in calories or joules. In the Sl system (the international standard of the system of units), heat energy is measured in joules, and the symbol used to represent 'joule' is 'J'.
 |
Download the notes
Chapter Notes: Temperature & Heat
|
Download as PDF |
Modes of Heat Transfer
- Heat is transferred from one region to another through three modes: conduction, convection, and radiation.
- Conduction is the primary mode of heat transfer in solids, and it occurs by collisions of neighbouring particles of the body.
- Convection occurs in fluids (liquids and gases) and is the transfer of heat by the movement of fluids.
- Radiation is the transfer of heat through electromagnetic waves and does not require a medium.
Conduction of Heat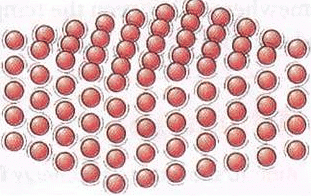
- Conduction is the transfer of heat through physical contact between two objects.
- The transfer of heat by conduction can occur within a body from one end to the other.
- The particles in a solid are tightly packed, and heat causes the particles to vibrate faster and transfer energy to neighbouring particles.
- Materials that allow heat to pass through them easily are called conductors, while materials that do not allow much heat to pass through them are called insulators.
- All metals are good conductors of heat due to their loose, free-moving electrons.
Conductors and Insulators of Heat
- In an activity to demonstrate the rate of conduction of heat in different metals, metal balls are glued on aluminium, iron, and copper rods, and the rods are arranged and heated using a burner.
- The metal balls start dropping due to the melting of wax, and it is observed that the rate of conduction of heat is highest for copper and lowest for iron.
- Some materials such as wood, straw, clay, rubber, glass, and bakelite are insulators and do not conduct heat very well.
- Air and water are also good insulators, and some materials such as wool, fur, and bird feathers become better insulators by trapping air between their fibres.
Conduction
Heat transfer through a material by direct contact of its molecules. Practical applications: use of good conductors like metals for cooking utensils, heat-resistant plastic for handles, copper bottom for stainless steel vessels, etc. Use of bad conductors like wool to retain body warmth.
Convection
Heat transfer in fluids (liquids and gases) through mass movement of their molecules. Practical applications: warming a fluid from below, placing a room heater at a lower level, placing an air conditioner at a higher level, designing windows and ventilators for cooling in summers, etc.
Radiation
Heat transfer through electromagnetic waves without any medium between the objects. Practical applications: heating by the sun, use of infrared heaters, etc.
Practical Applications of Conduction:
- Good conductors (like metals) are used for cooking utensils.
- Heat-resistant plastic is used for handles of vessels.
- Copper bottom is used for stainless steel vessels to make heating more efficient.
- Bad conductors (like wool) are used to retain body warmth.
Practical Applications of Convection
- Warming a fluid from below.
- Placing a room heater at a lower level to warm the room evenly.
- Placing an air conditioner at a higher level to cool the room evenly.
- Designing windows and ventilators for cooling in summers.
Radiation of Heat

- Heat energy can travel in the form of waves, known as electromagnetic waves.
- All bodies give out energy that travels in the form of radiation, just like light.
- The amount of heat energy that is absorbed by a body depends on its color.
- Bodies that are black absorb more radiated heat than white bodies.
- Black and dark bodies radiate more heat than light-colored bodies.
Practical Applications of Radiation
- Heat radiations can travel in vacuum and air, similar to light rays.
- Electric room heaters have mirrors behind the heating coil to reflect heat radiated to the front.
- Solar panels used for heating water have black metal sheets to increase heat absorption.
Preventing Loss of Heat
- Thermos flask as an example of minimizing heat loss by all three modes of heat transfer.
- Insulating materials (like plastic) used for the outer casing and cap to minimize heat loss by conduction.
- Double-walled bottle made of glass or stainless steel with vacuum between two walls to minimize heat loss by conduction and convection.
- Surface of the jar made highly reflective to minimize heat loss by radiation.
Key Words
Activity:
The teacher can conduct an activity to help students understand the concept of parasitic plants. The activity can involve the following steps:
Aim: To understand the concept of parasitic plants and their impact on other plant species.
Materials needed: Pictures of different parasitic plants, chart paper, markers.
Method:
- The teacher can show pictures of different parasitic plants to the students and ask them to identify the characteristics that make them parasitic.
- The teacher can then divide the class into groups and assign each group a different parasitic plant.
- Each group can create a chart paper presentation on their assigned parasitic plant, including its characteristics, host plant, and impact on other plant species.
- The groups can then present their charts to the class and discuss the similarities and differences between the different parasitic plants.
Observation:
The teacher can observe the students' participation in the activity and their understanding of the concept of parasitic plants. The teacher can also ask follow-up questions to assess the students' comprehension.
|
140 videos|108 docs|18 tests
|