Cyclic Hemiacetals and Hemiketals: A Fascinating Journey into Ring Formation | Organic Chemistry for MCAT PDF Download
| Table of contents |

|
| Introduction |

|
| Formation of Cyclic Hemiacetals and Hemiketals |

|
| Glucose: A Marvel of Cyclic Hemiacetal Formation |

|
| Fructose: A Journey into Hemiketal Cyclization |

|
Introduction
Cyclic hemiacetals and hemiketals are intriguing compounds that arise from the reaction between alcohols and aldehydes/ketones. This article will delve into the formation and stability of these cyclic structures, as well as explore specific examples such as glucose and fructose. So, let's embark on this captivating journey!
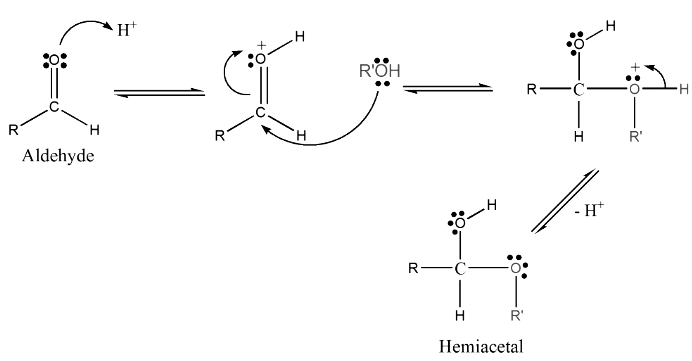
Formation of Cyclic Hemiacetals and Hemiketals
- Before we immerse ourselves in the world of cyclic hemiacetals and hemiketals, let's briefly recap their formation process. These compounds are created when an alcohol oxygen atom adds to the carbonyl carbon of an aldehyde or a ketone. This reaction occurs through the nucleophilic attack of the hydroxyl group at the electrophilic carbonyl group. However, since alcohols are weak nucleophiles, the attack on the carbonyl carbon is usually facilitated by protonation of the carbonyl oxygen.
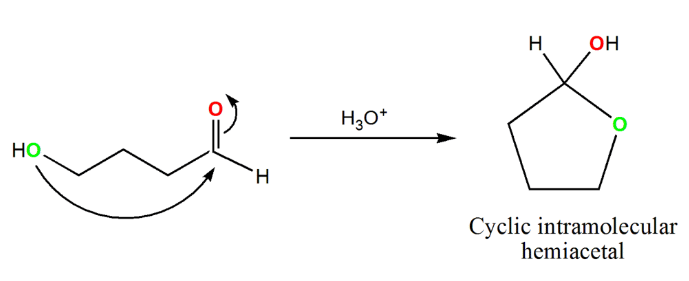
- When this reaction takes place with an aldehyde, the resulting product is called a "hemiacetal," whereas when it occurs with a ketone, the product is referred to as a "hemiketal." It is important to note that intermolecular hemiacetals/hemiketals, formed between separate molecules, are inherently unstable and tend to favor the original aldehyde or ketone. However, molecules containing both an alcohol and a carbonyl group have the potential to undergo an intramolecular reaction, leading to the formation of a cyclic hemiacetal/hemiketal. In contrast to their intermolecular counterparts, these cyclic structures are more stable. The stability of cyclic hemiacetals/hemiketals is greatly influenced by the size of the ring, with 5- and 6-membered rings being generally preferred. This type of intramolecular ring formation is frequently encountered in the realm of sugar chemistry.
Glucose: A Marvel of Cyclic Hemiacetal Formation
Let's take a closer look at the fascinating case of glucose. Starting with the Fischer Projection of glucose (C₆H₁₂O₆), which features an aldehyde group and five hydroxyl groups, we can clearly see its potential for intramolecular cyclic hemiacetal formation. The question arises: Why doesn't the hydroxyl group attached to C-4 react with the carbonyl group? Instead, the carbonyl group interacts with the hydroxyl group attached to C-5. The reason behind this lies in the resulting ring size. The attack of the C-4 hydroxyl group on the carbonyl group would lead to the formation of a 5-membered ring, whereas the attack of the C-5 hydroxyl group generates a 6-membered ring, which is thermodynamically more stable. Hence, the formation of a 6-membered ring is favored in the case of glucose.
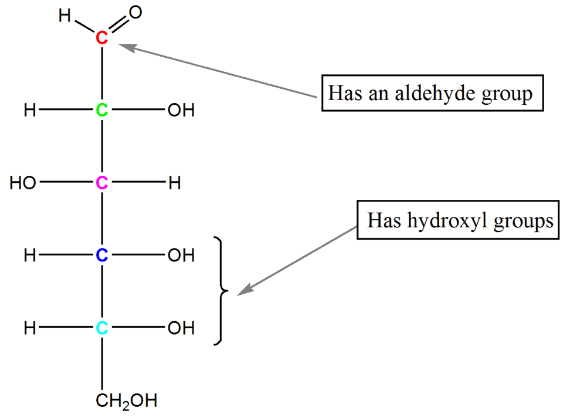
- Moving on to the hemiacetal of glucose in the Haworth Projection, we observe the creation of a new stereogenic center, C-1, known as the anomeric carbon. Glucose can exist in two isomeric forms, referred to as α and β, depending on the orientation of the OH group attached to the anomeric carbon (C-1) relative to the CH2OH group. These two forms are known as anomers of glucose. Notably, when transitioning from a Haworth Projection to a chair conformation, the groups pointing upwards in the former become equatorial in the latter, while the groups pointing downwards become axial.
- In an aqueous solution, glucose is present in equilibrium between its open and closed forms. This equilibrium involves the rotation of the C-1 → C-2 bond during the conversion from the closed to open form and vice versa. As a result of this mutarotation process, which encompasses the opening of the ring, rotation of the C-1 → C-2 bond, and subsequent closure of the ring, both α and β anomers coexist in solution. In the case of glucose, the β anomer is more prevalent than the α anomer, although this ratio may differ for other monosaccharides.
Fructose: A Journey into Hemiketal Cyclization
- Now, let's shift our focus to fructose and apply the same principles we explored with glucose. Fructose possesses a ketone group and five hydroxyl groups, making it capable of undergoing intramolecular hemiketal cyclization. Interestingly, fructose can cyclize in two different ways.
- In the first scenario, the hydroxyl group attached to C-5 attacks the carbonyl group, resulting in the formation of a 5-membered ring, known as the furanose form. On the other hand, in the second scenario, the hydroxyl group attached to C-6 attacks the carbonyl group, yielding a 6-membered ring, referred to as the pyranose form.
Conclusion
Cyclic hemiacetals and hemiketals provide us with a captivating glimpse into the world of ring formation in organic chemistry. From the stability influenced by ring size to the intriguing case studies of glucose and fructose, these compounds demonstrate the complexity and beauty of chemical reactions. Exploring their formation and behavior offers a deeper understanding of the intricate processes occurring at the molecular level. So, next time you encounter these cyclic structures, you'll have a newfound appreciation for their significance in the chemical world.
|
140 videos|5 docs|15 tests
|



















