Intramolecular and intermolecular forces | General Chemistry for MCAT PDF Download
Introduction
Understanding the intricate forces that govern the behavior of molecules is crucial for comprehending the physical properties and interactions that occur at the molecular level. In this article, we will delve into the captivating realm of intramolecular and intermolecular forces. By visualizing the analogy of sewing together towels, we will explore the disparity between these forces and their impact on the properties of compounds. So, let's embark on this enlightening journey!
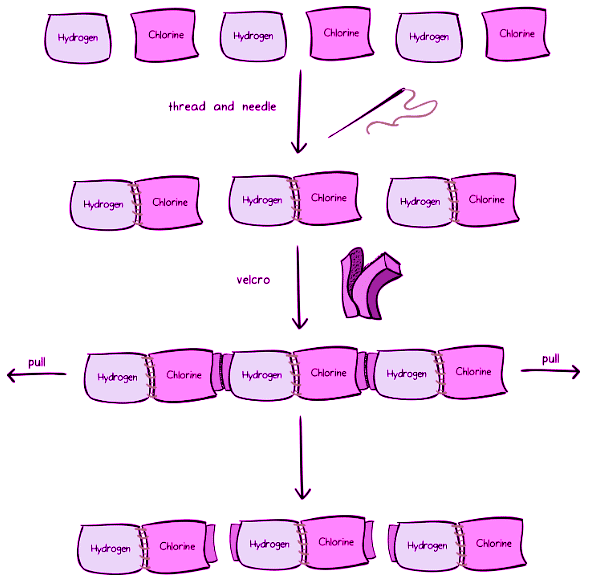
Towel Assembly: Unveiling Intramolecular and Intermolecular Forces
Imagine a collection of towels, some purple (labeled hydrogen) and some pink (labeled chlorine). Armed with a sewing needle and black thread, we sew one hydrogen towel to one chlorine towel, resulting in three pairs of towels: hydrogen sewed to chlorine. To further bind these pairs, Velcro is employed. The outcome is six towels interconnected through both thread and Velcro.
Unmasking the Strength: Intramolecular Forces
When we pull this assembly from both ends, the Velcro junctions succumb to the force, while the sewed junctions remain intact. This discrepancy unveils that the attachment created by Velcro is considerably weaker than the sewed junctions. Similarly, molecules possess intramolecular forces that hold their constituent atoms together.
Polar Covalent Bonds: Threads of Molecular Unity
Each hydrogen chloride molecule consists of hydrogen and chlorine atoms bound to each other through a polar covalent bond—analogous to the thread in our towel example. This bond represents a strong intramolecular force as electrons are shared unequally, creating partial charges. Polar covalent bonds are stronger than intermolecular forces.
Dipole-Dipole Attractions: Molecular Velcro
Moving beyond individual molecules, dipole-dipole attractions come into play. Analogous to the Velcro used in our towel assembly, dipole-dipole interactions occur between partially charged ions in neighboring hydrogen chloride molecules. These intermolecular forces, although weaker than polar covalent bonds, contribute to the overall molecular stability.
Defining the Forces: Intramolecular vs. Intermolecular
To establish a clear distinction, we define the two forces as follows:
- Intramolecular Forces: Forces that hold atoms together within a molecule.
- Intermolecular Forces: Forces that exist between molecules.
Decoding the Forces: Types and Relative Strengths
Now that we grasp the essence of intramolecular and intermolecular forces, let's explore their various types and understand their relative strengths.
A. Intramolecular Forces of Attraction
Intramolecular forces determine the nature and stability of molecules. They can be categorized into the following types:
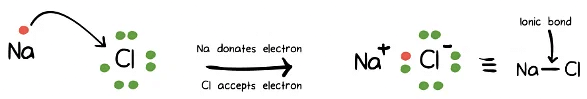
- Ionic Bonds: The Electrostatic Symphony
- Ionic bonds arise from the complete transfer of valence electrons between atoms, resulting in the formation of oppositely charged ions. This type of bond is present in ionic compounds, where metal cations lose electrons and nonmetal anions gain them.
Covalent Bonds: Sharing for Stability
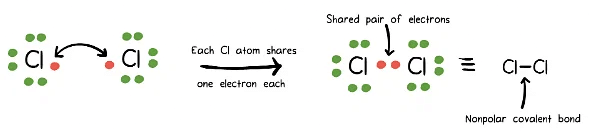
Covalent bonds form when atoms with similar electronegativities, denoting their affinity for electrons, share electrons to achieve stability. They can be further classified as:
- Nonpolar Covalent Bonds: Shared electrons between identical atoms or those with minimal electronegativity differences.
- Polar Covalent Bonds: Shared electrons between atoms with slightly different electronegativities, resulting in partial charges.
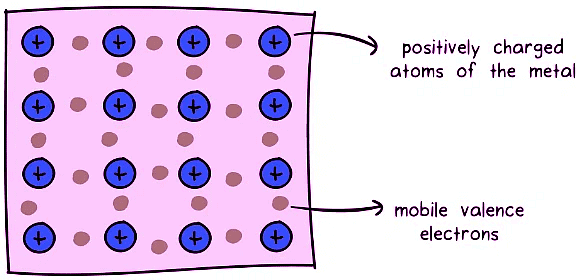
Metallic Bonding: The Orchestra of Electrons
Specific to metals, metallic bonding occurs through the delocalization of valence electrons within a metal lattice. The attraction between mobile electrons (sea of electrons) and fixed metal ions creates a cohesive force.
Intermolecular Forces of Attraction
While intramolecular forces are essential for molecular stability, intermolecular forces greatly influence the physical properties of compounds. Let's explore their types:
Dipole-Dipole Interactions: Dancing with Opposites
Dipole-dipole interactions manifest when partially positive regions of one molecule interact with partially negative regions of a neighboring molecule. These forces require partially charged ions and are the strongest among intermolecular forces.
Hydrogen Bonding: A Special Attraction
Hydrogen bonding is a unique form of dipole-dipole interaction occurring specifically between a hydrogen atom bonded to oxygen, nitrogen, or fluorine. The partially positive hydrogen attracts the partially negative counterpart in another molecule. These bonds are relatively strong and require significant energy to break.
London Dispersion Forces: The Invisible Tug
Under the umbrella of van der Waals forces, London dispersion forces are the weakest intermolecular forces. They exist between all types of molecules, regardless of polarity, and arise from temporary or induced dipoles. The more electrons a molecule possesses, the stronger the London dispersion forces become.
Impact on Compound Properties
The strength of intermolecular forces plays a pivotal role in determining the physical properties of compounds. Let's explore how these forces affect boiling and melting points:
- Ionic Compounds: With ion-to-ion attraction and London dispersion forces, ionic compounds exhibit the highest boiling and melting points among compounds.
- Covalent Compounds with Hydrogen Bonds: The presence of hydrogen bonds and London dispersion forces gives rise to relatively high boiling and melting points.
- Polar Covalent Compounds: Dipole-dipole attractions and London dispersion forces result in moderate boiling and melting points.
- Nonpolar Covalent Compounds: Solely relying on London dispersion forces, nonpolar covalent compounds have the lowest boiling and melting points.
Conclusion
Intramolecular and intermolecular forces intricately shape the behavior of molecules and compounds. By understanding these forces, scientists can unravel the mysteries of chemical reactions, molecular interactions, and physical properties. The captivating interplay between intramolecular and intermolecular forces continues to inspire scientific exploration and innovation, opening doors to new frontiers of knowledge.
|
164 videos|11 docs|16 tests
|















