Principles of Chromatography: Exploring the Stationary Phase | General Chemistry for MCAT PDF Download
Introduction
Chromatography, an essential analytical technique, serves as a powerful tool for separating complex mixtures into individual components. Whether it's liquid chromatography, gas chromatography, ion-exchange chromatography, or affinity chromatography, all these methods operate on the same fundamental principles. As an organic chemist who frequently conducts chromatographic separations, I stumbled upon a captivating pictorial representation of an actual separation I performed in the lab. This image serves as an excellent starting point for this enlightening exploration of chromatography's stationary phase.
Understanding the Chromatographic Process
Before diving into the intricacies of the stationary phase, let's familiarize ourselves with some key terms used in chromatography:
- Mobile phase or carrier: The solvent that moves through the column.
- Stationary phase or adsorbent: The substance that remains fixed inside the column.
- Eluent: The fluid entering the column.
- Eluate: The fluid exiting the column, collected in flasks.
- Elution: The process of washing out a compound through a column using a suitable solvent.
- Analyte: The mixture whose individual components require separation and analysis.
The central principle of chromatography involves the differential affinities and separation of analyte components based on their adsorption and solubility properties. To illustrate this process, let's visualize a column chromatographic separation setup:
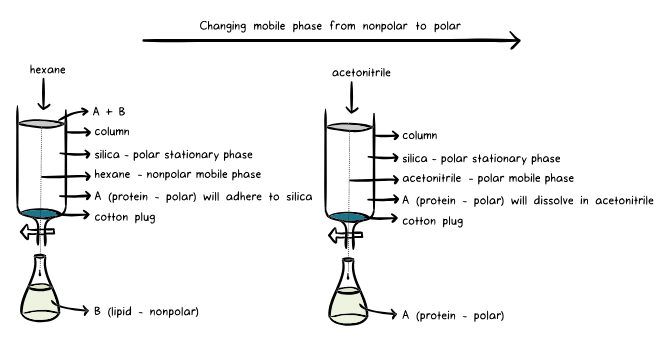
In this setup, the analyte is loaded onto a bed of silica, which acts as the stationary phase within the column. The solvent, known as the mobile phase, flows through the silica bed under the influence of gravity or pressure. As the solvent traverses the stationary phase, the various components of the analyte exhibit different degrees of adhesion to the silica, resulting in distinct bands of separation. Components with stronger adsorption to the stationary phase move more slowly, while those with weaker adhesion travel at faster rates. This differential movement leads to the separation of components within the analyte mixture. Analytical chromatography enables the purification of compounds ranging from milligram to gram scale.
A Simple Experiment: Unveiling the Power of Chromatographic Separation
To showcase the efficacy of chromatographic separation, let's conduct a straightforward experiment:
- Crush a few leaves in a mortar.
- Spot a drop of the leaf extract on a strip of chromatographic paper, approximately 0.5 cm above the edge.
- Place the paper strip in a jar containing a small volume of propanone (acetone), ensuring the edge of the paper comfortably dips into the solvent.
- Cover the jar with a lid to prevent solvent evaporation.
- Allow the solvent to rise up the paper through capillary action.
- Remove the paper strip from the jar once the solvent reaches the "solvent front" level.
What do you expect to observe? Remarkably, the various components of the leaf pigment separate, revealing the presence of numerous compounds within a single pigment. This demonstration highlights the power and complexity of chromatographic separation.
Principle of Separation: Adsorption and Solubility
- The separation of different components within an analyte mixture stems from their varying affinities toward the stationary and mobile phases. Affinity is governed by two key properties: "adsorption" and "solubility."
- Adsorption refers to how strongly a component of the mixture adheres to the stationary phase, while solubility denotes how readily a component dissolves in the mobile phase. A higher adsorption to the stationary phase results in slower movement through the column, while greater solubility in the mobile phase leads.
|
164 videos|11 docs|16 tests
|















