What are S-Block Elements?
S-block elements refer to elements that have their last valence electron present in the s-suborbital. They can be categorized into two groups: alkali metals, which have only one electron in their s-orbital, and alkaline earth metals, which have two electrons filling their s-orbital. The distribution of electrons in an atom follows the order of increasing energy, with the last electron potentially occupying the s, p, d, or f subshells.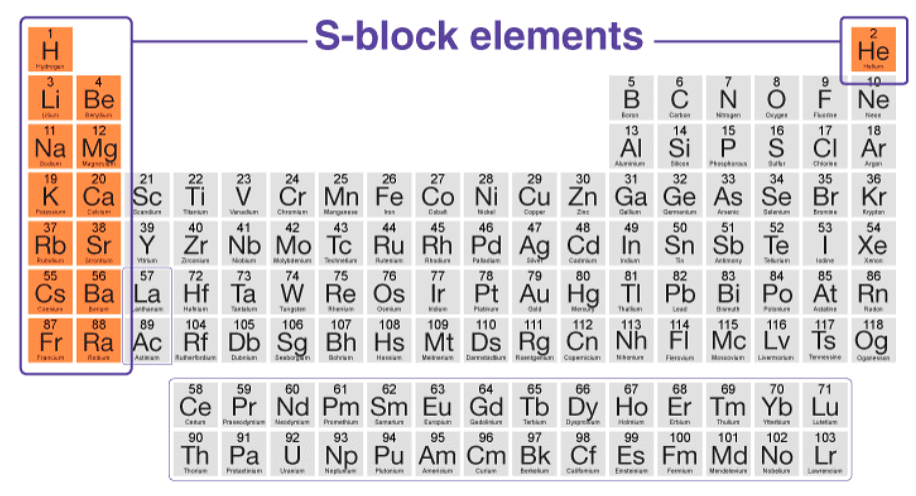
Electronic Configuration of S-Block Elements
The electronic configuration of S-block elements is explained below.
The alkali elements in the s-block consist of a single valence electron in their outermost shell. This outermost electron is loosely held, which makes these metals highly electropositive. Due to this, they are not available in a free state in nature. The general electronic configurations of s block elements – group 1 are as shown in the table below: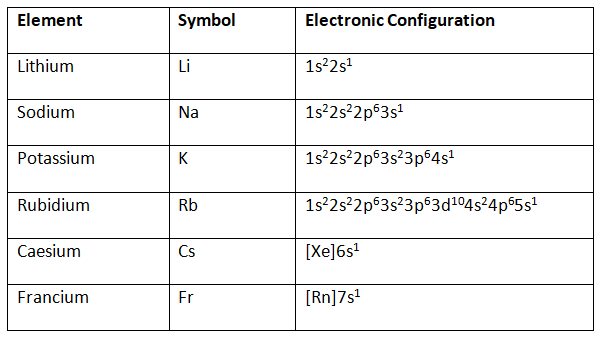
The electronic configurations of elements included in group 2 of S block elements are shown below:
Properties of S-Block Elements
Both alkali and alkaline earth elements exhibit a consistent pattern in their properties within their respective groups. However, the initial elements of both groups, Lithium and Beryllium, deviate significantly from the remaining elements within their groups. Interestingly, these first elements bear a resemblance to the diagonal element located in the adjacent column.
The anomaly of these S-block elements is due to the following:
- Low atomic and ionic size
- Greater charge density (charge/volume of the atom)
- Greater polarization
- Absence of d-orbitals.
Greater polarization of s block elements makes the first element more covalent and differentiates them from the rest, which is ionic.
The similarity in size and charge density makes them resemble the element diagonally placed in the next group (diagonal relationship).
It is observed that the physical and chemical properties of these s block elements change in a particular trend as the atomic number of the elements increases. Changes in the various properties of the group are as mentioned below:
Chemical Properties of s Block Elements
Atomic and Ionic Radii
When examining the s block elements in the periodic table, it becomes apparent that the alkali metals possess a larger size compared to other elements within the same period. This can be attributed to the fact that as the atomic number increases, there is a corresponding increase in the total number of electrons and the addition of electron shells. Consequently, when moving down the group, the atomic number increases, leading to an expansion in both the atomic and ionic radius of the alkali metals.
Ionization Enthalpy
When descending a group in the periodic table, the atomic size expands, leading to a reduction in the attractive force between the nucleus and the electrons in the outermost shell. Consequently, this decrease in attraction causes a decline in the ionization enthalpy. The ionization enthalpy of alkali metals, in particular, is notably lower compared to that of other elements.
Hydration Enthalpy
With an increase in the ionic sizes of elements, there is a corresponding decrease in hydration enthalpy. When the ion size is smaller, the hydration enthalpy is higher because the ion can accommodate a larger number of water molecules around it due to its high charge-to-radius ratio. As a result, the smaller ion becomes more hydrated.
Physical Properties of s Block elements
- In the s block elements, the density of the alkali metals increases down the group. Exception: the density of potassium is less than the density of sodium.
- Alkali metals have a low melting and boiling point due to weak metallic bonding.
- Alkali metals and their respective salts have the capability to impart colour to the oxidizing flame due to the heat generated from the flame, which excites the valence electrons from one energy level to another energy level. This helps in the detection of alkali metals during the flame test.
Diagonal Relationship within s Block Elements
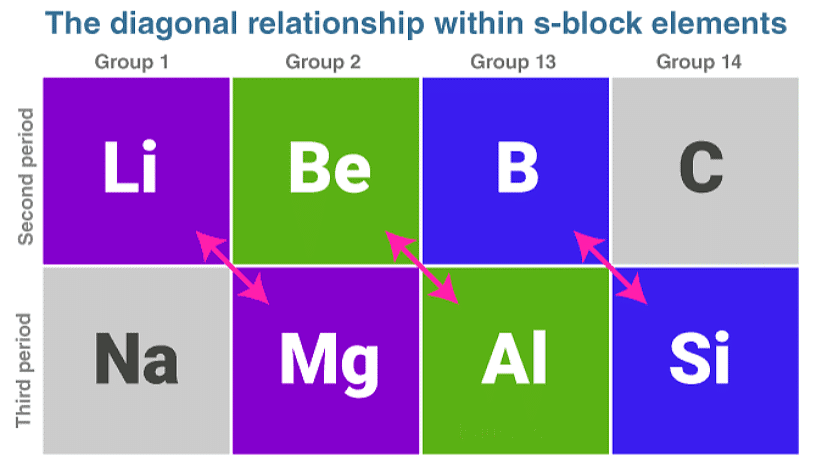
A diagonal relationship in s block elements exists between adjacent elements, which are located in the second and third periods of the periodic table. For example, the lithium of group 1A and the second period shows similarities with the properties of magnesium, which are located in the 2nd group and 3rd period.
Similarly, the properties of beryllium, which are located in the 2nd group and 2nd periods, show a likeness with the properties of aluminium which is located in the third period and third group. The two elements which show similarities in their properties can be called diagonal pairs or diagonal neighbours.
The properties of s block elements vary significantly when compared to the other elements of the sub-group they belong to. The diagonal neighbours show a lot of similarities. Such a relationship is exhibited as you move left to the right and down the group; the periodic table has opposing factors.
For example, the electronegativity of the s block elements increases as we go across the period and decreases as we go down the group. Therefore, when it is moved diagonally, the opposite tendencies cancel out, and the value of electronegativity almost remains the same.
Similarities between Lithium and Magnesium
- The hardness of lithium and magnesium is higher than the other elements in their respective groups.
- Chlorides of lithium and magnesium have the capability to be soluble in ethanol.
- They are lighter when compared to other elements in their groups.
- Lithium and magnesium react gently with water. The oxides and hydroxides are less soluble.
- In the presence of nitrogen, lithium and magnesium form their respective nitrides.
- Superoxides are not formed when lithium and magnesium react with excess oxygen.
- Carbon dioxide and its respective oxides are formed when carbonates of magnesium and lithium are heated.
Similarities between Beryllium and Aluminium
- Aluminium hydroxide and beryllium hydroxide react with excess alkali to form their respective ions.
- Both these elements have the capacity to withstand the acid attack due to the presence of an oxide film on the surface of the metal.
- Both these metals have the tendency to form complexes.
- Chlorides of both these metals possess the capacity to be soluble in organic solvents.

|
Explore Courses for JEE exam
|

|
















